ACHAIKI IATRIKI | 2023; 42(3):123–126
Editorial
Theodora Katsila1, Vivi Bafiti1, Dimitrios Kardamakis2
1Institute of Chemical Biology, National Hellenic Research Foundation, Athens, Greece
2Department of Radiation Oncology, Department of Medicine, School of Health Sciences University of Patras, Patras, Greece
Received: 22 May 2023; Accepted: 10 Jul 2023
Corresponding author: D. Kardamakis, Department of Radiation Oncology, University of Patras Medical School, Patras, Greece, E-mail: kardim@upatras.gr
Key words: Radiation oncology, biomarkers, translational precision medicine
INTRODUCTION
In the past 50 years, the subject of radiation oncology has expanded enormously, not only in the fields of treatment delivery technology (stereotactic techniques) and anatomical imaging of structures of interest (adaptive radiotherapy), but also in the incorporation of hybrid whole-body imaging modality (positron emission tomography, PET/computed tomography, CT) in the radiation treatment planning and the integration of molecular profile data of the tumour and the normal tissues in decision-making [1-3]. In modern oncology, significant progress has been made in prescribing the molecular characteristics of cancerous or normal cells, along with imaging data, serving as biomarkers with the hype to better inform clinical practice [4,5]. Radiation oncologists have been actively involved in the exploration and implementation of biomarkers in their field.
Molecular and image-based biomarkers in radiation oncology
Nowadays, molecular biomarkers are part of the comprehensive cancer patient care, allowing the prognosis of the disease, predicting patient response to a particular therapy, and allowing clinicians to apply personalised medicine [6,7]. Image-based biomarkers are also actively discussed. To name but a few: tumour size, shape, texture and volume; apparent diffusion coefficient; standardised uptake value; blood flow and perfusion; volumetric modulated Arc therapy parameters; and hypoxia imaging inform about tumour characteristics, treatment response and treatment-related toxicities [8,9]. As imaging techniques and analysis methods are emerging, the landscape of image-based biomarkers in radiation oncology continues to expand, providing opportunities for improved treatment planning, response assessment and personalised care.
The concept of Translational Precision Medicine calls for the application of molecular and digital (i.e., image-based) biomarkers in a way that is feasible and clinically relevant for clinical trials, accepted by regulators and of note, patients [10]. Even though molecular and image-based biomarkers refer to an objective medical state that can be measured with accuracy and reproducibility [11,12], unfortunately in real-world situations, several challenges are still to be overcome when combining molecular and image-based data with radiation oncology routine practice [4,13]. To this extent, artificial intelligence (AI) undeniably holds remarkable potential for revolutionising the field of radiation oncology in the coming years. The integration of AI into this domain has already showcased promising advancements, empowering radiation oncologists with enhanced precision, efficiency, and personalised treatment approaches [14]. By leveraging machine learning algorithms and data analytics, AI can assist in the identification and utilisation of biomarkers, both molecular and image-based ones, thereby facilitating improved patient risk stratification, treatment planning, and therapy response assessment. Furthermore, AI-driven technologies have the capacity to optimise treatment delivery, enabling real-time adaptation and ensuring optimal tumour targeting while minimising damage to surrounding healthy tissues. As the research and development in this area continue to unfold, embracing AI’s capabilities and exploring its potential in radiation oncology will undoubtedly pave the way for innovative solutions, better patient outcomes, and the constant evolution of this vital medical discipline.
Are radiation oncologists ready for prime time?
As the field of biomarkers continues to advance, radiation oncologists play a critical role in incorporating these tools into their practice to enhance patient care and treatment outcomes. For the practicing radiation oncologist, a series of challenges arise from technical, clinical and logistical aspects.
Long waiting times for the results. The period between the referral of the patient and the results from the molecular analysis can delay the initiation of the therapy, putting the patient at an extra risk for relapse in cases of adjuvant treatment. Although we know that the association between a delay in starting radiation therapy (RT) and the outcome is complex and does not harm all patients waiting for RT [15], timely decision-making remains key. For the radiation oncologist to have the time to think, decide and act, a supporting framework of administrative and health professionals and digital tools and technologies alike shall be available for longitudinal assessment and follow-up.
Insufficient tumour and/ or normal tissue specimen for analysis. This is a real drawback for incorporating molecular analysis in the therapeutic algorithm. Radiation oncologists need to know the molecular profile of the normal tissues as well to predict the appearance and the severity of side effects. On the other hand, regarding the well described phenomenon of tumour heterogeneity, the molecular analysis of an insufficient tumour specimen may not represent the “true molecular profile”. In any case, there are biomarkers that are considered as surrogates, as the information they provide cannot be used as the single variable defining disease type, staging, and response to RT. For image-based biomarkers, appropriate use must be based on knowledge of the relationship to the underlying biological processes, the physical origin of the biomarker signal as well as their strengths and limitations [16].
Results are presented in reports in a way not familiar to clinicians. This problem can be surpassed by using a template in reporting the results to the health care team in a way that fosters clinical significance. At the same time, oncologists need to obtain a basic level of understanding such molecular analysis reports by upgrading their training curriculum and putting emphasis on the molecular biology of cancer in their continuing medical education activities. Educational resources that are updated based on white papers and clinical guidelines describing molecular and image-based biomarkers, the techniques used to quantify them, their strengths and weaknesses within the context of their application to radiation oncology so as to ensure their appropriate use and application are vital. Here, the well-established roles of multidisciplinary tumour boards and working groups are extremely important in helping oncologists to read and interpret molecular and image analysis results [17-19].
Clinical utility is an old problem in oncology, yet unsolved. Data from in vitro and in vivo preclinical studies are difficult to be transferred to clinical situations. Translating biomarker findings into actionable clinical decisions can be complex. Radiation oncologists need to understand the clinical significance of biomarkers, if any, plus their limitations and how to integrate them with individual patient characteristics, treatment modalities and response patterns [20,21].
Most published data originate from chemotherapy studies and refer to biomarkers involving a “single gene” – “single drug” association. In the translational precision medicine era, the biomarker field is shifting toward biomarker signatures addressing best disease complexity and population differences. From such biomarker signatures, biomarker panels and companion diagnostics often arise. In radiation oncology, such efforts include the field of radiogenomics that involves studies on assessing gene – radiation effect relationships and radiomics, in which quantitative features from medical images are extracted and analysed [22]. Coupling molecular to image-based biomarkers holds promise easing integration into clinical workflows [8].
Lack of clear guidelines connecting the molecular biology and image findings with the clinical status of the patient and the stage of the disease. In modern radiation oncology practice, efforts are being made to individualise radiation delivery on the basis of genes associated with tumour and normal tissue radiosensitivity [23]. Same for proteins and lately, metabolites plus images. Standardisation is key for reproducibility, ensuring consistency and quality assurance measures among clinical settings [4].
The power of synergy
In summary, even though recently published surveys state that oncologists in general show a low self-esteem in integrating molecular analysis data and/or image data in clinical data, our personal beliefs are that radiation oncologists are ready to embrace biomarkers and hence, play an important role in advancing the field of precision oncology if they gain additional knowledge in areas such as molecular biology and bioinformatics and collaborate more closely with molecular biologists, biomedical engineers, bioinformaticians and pathologists [4,18,22-23]. Radiation oncologists need a support network of administrative personnel and health professionals as well as tumour boards and working groups for their translational knowledge continuum (Figure 1).
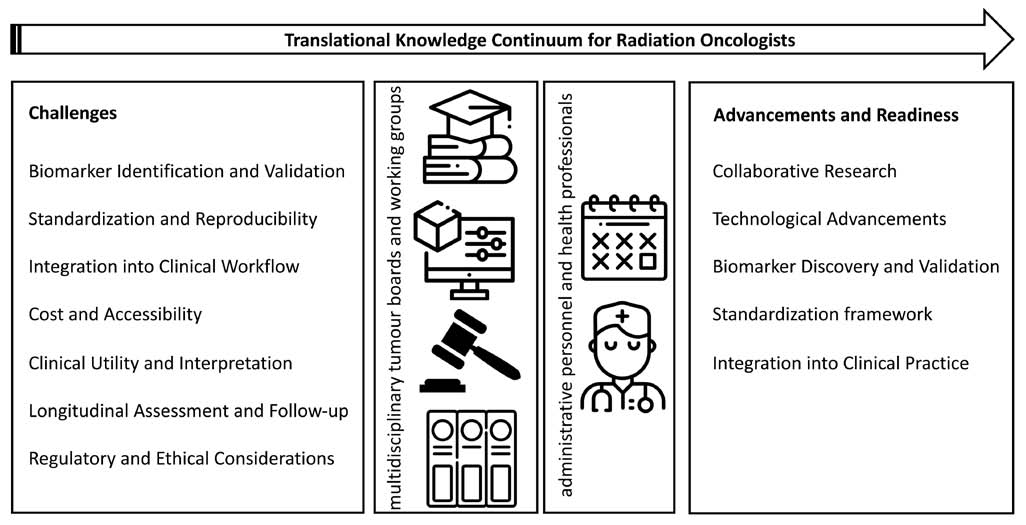
Figure 1. Radiation oncologists are ready to embrace translational biomarkers. Key challenges are clearly defined and hence, tumour boards and working groups are collectively putting forward implementation schemes. Such schemes may be translated in clinical routines if supported by administrative and health professionals that support radiation oncologists toward decision- and sense-making.
Conflict of interest: None to declare
Declaration of funding sources: None to declare
Author contributions: TK, VB: writing, data interpretation, review of the final draft of the article. DK: conception, writing, data interpretation, review of the final draft of the article
REFERENCES
- Fornell D. 7 trends in radiation therapy at ASTRO2021. Imaging Technology News (ITN). Retrieved from [https://www.itnonline.com/article/7-trends-radiation-therapy-astro-2021]
- Garibaldi C, Jereczek-Fossa BA, Marvaso G, Dicuonzo S, Rojas DP, Cattani F, et al. Recent advances in radiation oncology. Ecancermedicalscience. 2017;11:785.
- Papathanasiou N, Papadimitropoulos K, Spiliotopoulou M, Karagkouni E, Apostolopoulos D. PET/CT in the management of cancer patients. Achaiki Iatriki. 2022;41(3):135-45.
- McGee KP, Hwang K-P, Sullivan DC, Kurhanewicz J, Hu Y, Wang J, et al. Magnetic resonance biomarkers in radiation oncology: the report of AAPM task group 294. Med Phys. 2021;48(7):e697-32.
- Bratman SV, Milosevic MF, Liu F-F, Haibe-Kains B. Genomic biomarkers for precision radiation medicine. Lancet Oncol. 2017;18(5):e238.
- Sarhadi VK, Armengol G. Molecular biomarkers in cancer. Biomolecules. 2022;12(8):1021.
- Scarborough JA, Scott JG. Translation of precision medicine research into biomarker-informed care in radiation oncology. Semin Radiat Oncol. 2022;32(1):42-53.
- Katsila T, Matsoukas MT, Patrinos GP, Kardamakis D. Pharmacometabolomics informs quantitative radiomics for glioblastoma diagnostic innovation. OMICS. 2017;21(8):429-39.
- Katsila T, Liontos M, Patrinos GP, Bamias A, Kardamakis D. The new age of -omics in urothelial cancer – Re-wording Its diagnosis and treatment. EbioMedicine. 2018;28:43-50.
- Hartl D, de Luca V, Kostikova A, Laramie J, Kennedy S, Ferrero E, et al. Translational precision medicine: an industry perspective. J Transl Med. 2021;19(1):245.
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89-95.
- Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463-6.
- Valentini V, Boldrini L, Mariani S, Massaccesi M. Role of radiation oncology in modern multidisciplinary cancer treatment. Mol Oncol. 2020;14(7):1431-41.
- Huynh E, Hosny A, Guthier C, Bitterman DS, Petit SF, Haas-Kogan DA, et al. Artificial intelligence in radiation oncology. Nat Rev Clin Oncol. 2020;17:771.
- Žumer B, Pohar Perme M, Jereb S, Strojan P. Impact of delays in radiotherapy of head and neck cancer on outcome. Radiat Oncol. 2020;15(1):202.
- Baumann M, Ebert N, Kurth I, Bacchus C, Overgaard J. What will radiation oncology look like in 2050? A look at a changing professional landscape in Europe and beyond. Mol Oncol. 2020;14(7):1577-85.
- FDA Biomarkers Working Group. U.S. Food and Drug Administration (FDA). Retrieved from https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/fda-biomarkers-working-group
- West HJ, Lovly CM. Ferrying oncologists across the chasm of interpreting biomarker testing reports: systematic support needed to improve care and decrease disparities. JCO Oncol Pract. 2023;OP2300010.
- Wallner PE, Anscher MS, Barker CA, Bassetti M, Bristow RG, Cha YI, et al. Current status and recommendations for the future of research, teaching, and testing in the biological sciences of radiation oncology: report of the American Society for Radiation Oncology Cancer Biology/Radiation Biology Task Force, executive summary. Int J Radiat Oncol Biol Phys. 2014;88(1):11-7.
- Uusijärvi H, Bernhardt P, Forssell-Aronsson E. Translation of dosimetric results of preclinical radionuclide therapy to clinical situations: influence of photon irradiation. Cancer Biother Radiopharm. 2007;22(2):268-74.
- Miebach L, Berner J, Bekeschus S. In ovo model in cancer research and tumor immunology. Front Immunol. 2022;13:1006064.
- Rosenstein BS, West CM, Bentzen SM, Alsner J, Andreassen CN, Azria D, et al. Radiogenomics: radiobiology enters the era of big data and team science. Int J Radiat Oncol Biol Phys. 2014;89(4):709-13.
- Bristow RG, Alexander B, Baumann M, Bratman SV, Brown JM, Camphausen K, et al. Combining precision radiotherapy with molecular targeting and immunomodulatory agents: a guideline by the American Society for Radiation Oncology. Lancet Oncol. 2018;19(5):e240-e251.