ACHAIKI IATRIKI | 2023; 42(4):190–200
Review
Ilias Haliassos1, Rafaella Argyriadi2, Despoina Spyropoulou1, Dimitrios Kardamakis1, Suzanne Abdullah2
1 Department of Radiation Oncology, University Hospital of Patras, University Campus, 26503, Patras, Greece
2 Department of Medical Oncology, University Hospital of Patras, University Campus, 26503, Patras, Greece
Received: 01 May 2023; Accepted: 28 Sep 2023
Corresponding author: Dimitrios Kardamakis, Department of Radiation Oncology, University Hospital of Patras, University Campus, 26503, Patras, Greece, E-mail: kardim@upatras.gr
Key words: Spinal cord compression, imaging; neurosurgery, radiotherapy, chemotherapy, corticosteroids, rehabilitation
Abstract
Metastatic acute spinal cord compression (MASCC) is a serious complication in oncologic patients, causing a remarkable impingement in the patient’s quality of life. Symptoms at presentation include sensory loss, pain, sphincter dysfunction and paralysis. It is an emergency that necessitates prompt diagnosis and immediate intervention from a multidisciplinary team to maintain neurological function and enhance the functional outcome given the risk of permanent injury. Imaging modalities such as contrast-enhanced Magnetic Resonance Imaging (MRI) and/or Computerised Tomography (CT) are of paramount importance for establishing the diagnosis. Ambulatory status at presentation is an important prognostic factor in these patients. The goals of treatment are preservation of ambulation, neurological
function and pain management. Rapid initiation of steroids followed by surgical decompression in selected patients, and radiation therapy (RT) are the main therapeutic approaches. Additionally, chemotherapy in specific tumour types, bisphosphonates and supportive care measures promote quality of life, alleviate symptoms, and prevent additional complications.
INTRODUCTION
Metastatic acute spinal cord compression (MASCC) is a devastating demonstration of a metastatic cancer lesion displacing and compressing the spinal cord or cauda equina within the spinal canal [1]. Most patients with MASCC (80%) have a history of cancer diagnosis, while in up to 20% of them, the diagnosis of a primary cancer is absent at the time of presentation [2]. Non-Hodgkin lymphoma, multiple myeloma, lung, prostate, breast cancer and cancer of unknown primary tumour (CUPT) are the most frequent malignancies causing MASCC [2–6]. Although approximately 30% of all oncologic patients develop metastases in the spine, about 10-20% of them develop spinal cord compression [7]. In total, 5% to 10% of all cancer patients experience spinal cord compression during the natural history of their disease [4,8]. Data from the USA show that the incidence is approximately 20,000 new cases and the median age at the time of diagnosis is 65 years [9,10].
Although MASCC potentially may implicate any portion of the spinal cord, it is more frequently located in the thoracic, lumbosacral, and cervical segments of the spine [11]. Thoracic and cervical spine are usually involved by breast cancer and lung metastases, while the lumbosacral spine is targeted by pelvic tumours, colon cancer and prostate cancer [12].
Extradural (or epidural) metastatic lesions are caused by direct extension from a metastasis in the body of a vertebra [8]. The culprit of the epidural compression is soft tissue in 75% of the cases and in the remaining 25%, compression is caused by collapsed bony fragments [13]. In approximately 5% of cases of all MASCCs, intradural (but extramedullary) metastases are caused by tumour cell migration via the circulatory movement of the cerebrospinal fluid [5,11]. Rarely, metastases from breast, lung and renal cancer are intramedullary [7].
Two major mechanisms seem to contribute to the development of neurological damage secondary to compression: At an early stage, tumour extension into epidural space causes compression of the Batson venous plexus which in turn increases vascular permeability with subsequent development of venous congestion, vasogenic oedema and demyelination. Recovery from neurological damage is still reversible if the compression is relieved. However, if compression is sustained, secondary vascular injury causes increased pressure on small arterioles, occlusion of these vessels and finally infarction of the cord structure, at which time the damage is irreversible with loss of neurological function [11,14,15].
The majority of MASCC patients die within the first year following diagnosis [9]. Morbidity from MASCC is associated with increased susceptibility to infections and thromboembolic events, which in turn reduce the survival [7]. A meta-analysis of 38 studies of MASCC paraplegic patients demonstrated one-year survival rates of 12-58%, with median survival between 2.4 and 30 months [16].
In this review, we provide evidence-based data on the clinical presentation, diagnosis, and management of MASCC. We emphasise the importance of a multidisciplinary team approach, including spine surgeons, radiation oncologists, medical oncologists, palliative care clinicians, physiotherapists, and psychologists.
CLINICAL PRESENTATION
Although the initiation of symptoms in this group of patients gradually spans over several days or even weeks, most of the times symptoms progress expeditiously, ranging from a few hours to a few days.
Mechanical, local or radicular pain is by far the primal and most constant symptom in almost 95% of all MASCC cases [6]. It originates from the direct compression of the spinal cord and/or the nerve roots from the metastatic lesion. It is of paramount importance to take into consideration the fact that if the compression progresses slowly, isolated pain may be the only tangible symptom without neurological manifestations present at that time. Localisation of the pain is dependent on the segment of the spinal cord affected. High intensity back pain reaching the level of 8-9/10 in the Visual Analogue Scale, aggravated by any movement or light physical activity, is suggestive of spinal instability [14,17]. The irritation or direct compression of nerve roots is responsible for radicular pain. It is distributed to the dermatome of the involved nerve root and is typically quantified as constant in duration (i.e., sciatic nerve pain). This type of pain is increased by straining and as such, a useful differential diagnosis tool is the application of straining maneuvers i.e., Valsalva, that intensify radicular pain [18].
Motor impairment is the second most frequent symptom [19,20]. Damage of the upper or lower motor neuron is manifested with classical neurological symptoms such as limb weakness, difficulty in walking, Babinski sign, hand coordination difficulty, brisk reflexes, and balance issues [21].
Furthermore, sensory loss is also typical. For example, loss of temperature and/or pain sensation, reflex loss, or radicular sensory loss, correlates reliably with the level of compression [5]. For cervical and thoracic epidural MASCCs, flexion of the patient’s neck elicits a pathognomonic abrupt sensation of electric shock running along the portions of the spine (Lhermitte’s sign).
Commonly occurring as a delayed symptom, is the development of autonomic dysfunction and loss of voluntary sphincteric control which causes bladder and/or bowel dysfunction (i.e., urinary bladder and/or fecal incontinence or retention) and is associated with a poorer prognosis [22].
An interesting although rare clinical variant of MASCC is the cauda equina syndrome. In adults, the spinal cord ends at the lower end of the L1 vertebra or at the upper end of L2 vertebra. Epidural metastases below that level manifest with flaccid paraparesis of feet and toes, absent ankle reflexes, impaired hip abduction and extension, saddle paresthesia and sphincter dysfunction [23].
DIAGNOSIS
The phrase ‘’time is the essence’’ fully applies in MASCC patients, regarding the diagnosis and treatment. Any delays can have dreadful implications on the ambulatory status and sphincter functions of the patient, leading eventually to deterioration in the quality of life and consequently reduced survival. Delays in obtaining the diagnosis are due to either the absence of recognition of the symptoms by the patient himself or the erroneous attribution by the attending physician of the referred symptoms to other pathologic entities.
Appropriate imaging modalities are essential for establishing the diagnostic hypothesis of MASCC, localising the level of spinal cord or cauda equina compression, quantifying the degree of compression and planning the treatment of MASCC [18].
The gold standard imaging study is gadolinium-DTPA contrast enhanced MRI with a sensitivity of 93% and a specificity of 98% [4]. In order not to misdiagnose MASCC present in multiple levels of the spinal cord, or metastases affecting non-symptomatic vertebrae, sagittal T1 and T2-weighted sequences, as well as Diffusion-Weighted images (DWi) of the entire spine should be obtained [24,25].
Complementary to the MRI, is CT scan with 3D-plane reconstruction which assesses spinal cord stability and assists the surgical planning (i.e., vertebroplasty, kyphoplasty or spinal surgery) [4]. It is nevertheless an option in cases where absolute contraindications performing MRI are present or MRI is inadequate. Even though CT scans permit superior depiction of bone tissue involvement within the spinal canal, myelography on the other hand is a time-consuming, labor intensive, more expensive and invasive procedure.
Another diagnostic modality to obtain first line images is plain X-ray. Its role before any treatment is to reveal dislocation of bony fragments from compression or pathologic fractures and areas of vertebral erosion, whereas its post-surgery function is to evaluate the structural integrity of the instrumentation as well as spinal alignment [2,7].
Additional imaging studies encompass positron emission tomography (PET) combined with CT and bone scan (99mTc-MDP). Both techniques are helpful in detecting vertebral metastases but remain inferior to MRI regarding the involvement of neural tissue and accurately localising the metastatic lesion [2].
TREATMENT
Therapeutic goals of MASCC are firstly, to conserve or recover the ambulatory status, and secondly the preservation or improvement of neurological function and analgesia. Treatment is largely palliative and only seldomly, in patients with MASCCs as the only site of disease (e.g., patients with renal cell cancer and no other systemic metastases), the intention of treatment is curative [26]. The processes of diagnosis and treatment should be closely associated and start and proceed hand in hand, as early intervention undoubtedly correlates with an improved outcome [27].
Surgery
To prevent unnecessary surgical morbidity, major surgical interventions should be considered for patients with a life expectancy of at least 3 months [28]. Various research groups have published scoring tools for the preoperative evaluation of a prognosis of a patient with a metastatic spinal tumour. Among these are the Tokuhashi score, the Spinal Instability Neoplastic Score (SINS) and the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), commonly referred to as the ASIA Exam.
The Tokuhashi score is a prognostic scoring system for spinal metastases. This system dates back in 1989. A revised version was published in 2005 [29]. The Spinal Instability Neoplastic Score (SINS) identifies patients who may benefit from surgical consultation or intervention. It also acts as a prognostic tool for surgical decision making [30]. The ASIA Exam, was developed by the American Spinal Injury Association (ASIA) as a universal classification tool for spinal cord injuries based on a standardised sensory and motor assessment, with the most recent revision published in 2019 (Figure 1) [31,32].
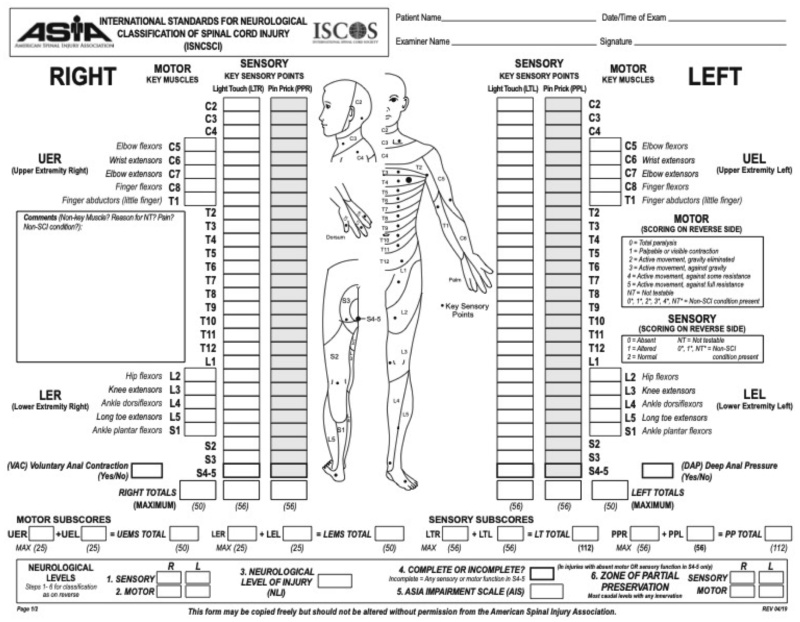
Figure 1. The ASIA exam.
Under permission: American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury, revised 2019; Richmond, VA.
The classical surgical approach for MASCC patients is the posterior decompressive laminectomy, which is associated with an increased rate of complications, such as spinal instability and wound infections to mention but a few [33]. Later, development of new anterior and lateral approaches widened the indications and enabled better access to the lesion site, circumferential decompression of the spinal cord as well as intraoperative reconstruction of the spine.
Several non-randomised surgical trials reported promising results with these novel surgical approaches. A meta-analysis of various cohort studies suggested better outcomes in patients who received surgery followed by radiotherapy [8]. This meta-analysis determined that patients who had undergone surgery had 1.3 times higher probability to be ambulatory post-surgery and two times more likely to recover ambulatory function than patients who had received radiation as monotherapy. Altogether, ambulatory success rates for surgical treatment were 85% and for radiation therapy 64%. The efficacy of direct decompressive surgery was assessed in a randomised multi-institutional trial [15]. This was the first randomised trial demonstrating the advantage of decompressive surgery followed by postoperative radiation over radiation alone. The primary endpoint was the ability to walk after treatment. All patients received high-dose dexamethasone. Both treatment groups received 30 Gy of radiation in 10 fractions. In the surgical group, more patients were able to walk after treatment (84% vs 57%; p = 0.001). Additionally, surgical patients retained the ability to walk longer (median 122 vs 13 days; p = 0.003) and regained the ability to walk more frequently than did those in the radiotherapy group alone (62% vs 19%; p = 0.01). Moreover, surgical treatment lowered the demand for corticosteroids and opioids and was correlated with lower 30-day morbidity than the radiotherapy-only group. Potential complications of surgery involve cerebrospinal fluid leak, thromboembolic disease, wound infection, vasogenic oedema, respiratory complications [34].
Modern surgical techniques allowing circumferential decompression of the spinal cord with synchronous stabilization and reconstruction techniques can be classified in five types: anterolateral transthoracic endoscopic approach usually through the pleural space, anterior transcavitary approach (retroperitoneal or transthoracic), posterolateral approach (lateral gutter: laminectomy plus removal of pedicle, transpedicular, costo-transversectomy, pedicle sparing transfacet approach), posterior approach (midline laminectomy) and lateral extra cavitary approach [33,35].
In patients with an anterior, anterior paraspinal, unilateral epidural tumour or vertebral body lesion in the lumbar and/or thoracic region, the most appropriate technique is the anterior transcavitary (retroperitoneal or transthoracic), delivering direct access to the vertebral body. When maximal circumferential decompression is necessary, the technique could be combined with a posterior or posterolateral approach. On the other hand, always in the context of metastatic lesions located in the lumbar or thoracic region, when there are contraindications for an anterior transcavitary approach or in cases of multilevel disease, major spinal deformity, circumferential compression of the spinal cord, diffuse bone involvement, the posterolateral approach is to be preferred to the anterior counterpart. Stabilization and reconstruction techniques consist of the use of expandable titanium cages, Steinmann pins, autologous bone graft and a synthetic resin produced from the polymerization of methyl methacrylate namely polymethylmethacrylate (PMMA) [35].
More advanced surgical techniques are currently developed that provide optimal decompression to the spinal cord. This is the case of the separation surgery, in which only a portion of the tumour is removed, creating a margin surrounding the spinal cord for the subsequent application of radiotherapy [36,37]. When the risk of massive intraoperative hemorrhage (such as in tumours with rich vascularisation e.g., leiomyosarcoma, hepatocellular carcinoma, renal cell carcinoma), surpasses the benefit of decompression, an efficient and safe technique to contain blood loss is the use of a minimally invasive image-guided procedure called trans arterial chemoembolisation [38,39].
In cases of malignant fractures, other minimally invasive image-guided treatment options include percutaneous kyphoplasty and percutaneous vertebroplasty [40–42]. In percutaneous vertebroplasty polymethylmethacrylate is directly injected into the vertebral body. This stabilises the fracture and relieves pain and disability. Kyphoplasty differs from vertebroplasty by adding an important additional step which is the placement of an expandable balloon to create a cavity which reduces kyphosis, restores vertebral body height and aligns the spinal canal and then injection of PMMA follows. Although the use of these minimally invasive procedures in epidural MASCCs is considered a relative contraindication, in poor surgical candidates the combination of radiation therapy along with the previously mentioned percutaneous techniques can considerably alleviate pain.
Radiation therapy
Palliative RT as a monotherapy is a treatment of choice for patients with an expected survival of 6 months or less. In this patient population surgical procedures should be avoided [37,43,44].
The most important predictive factor of RT outcome is the neurological status of the patient before irradiation, followed by the Karnofsky score, the duration of neurological symptoms, the interval between initial tumour diagnosis and MASCC, the extent of compression of the thecal sac and the histology of the primary tumour [45,46].
The standard treatment procedure of MASCCs for several years has been the combined action of radiation and steroids. Numerous prescription modalities have been used in clinical practice, without establishing superiority of any regimen over the others. The fractionation schedule, total and daily dose are yet subject of debate.
Typical conventional radiation treatment plans comprise total doses of 20 Gy in 5 fractions for the short course (offered to patients with shorter life expectancies), to 30 Gy in 10 fractions for the longer courses in those with less extensive disease [33].
Treatment is usually delivered with a single posterior field for the thoracic, lumbar, or sacral spine or two opposed lateral fields for the cervical spine.
The radiation field includes the involved spinal segment plus one level above and below this region [45]. Rades et al. investigated patients with MASCCs, treated with RT by short-courses i.e., one fraction of 8 Gy in 1 day, or 5 fractions of 4 Gy in 1 week, versus patients who underwent longer-courses of RT i.e., 20 fractions of 2 Gy in 4 weeks or 15 fractions of 2.5 Gy in 3 weeks or 10 fractions of 3 Gy in 2 weeks [47]. Similar outcomes were reported between all cohorts in terms of motor function improvement and ambulatory rates after treatment. A palliative treatment of a single dose of 8 Gy in 1 fraction is a rational approach for patients with a shorter life expectancy. On the other hand, longer courses i.e., 30 Gy in 10 fractions or 40 Gy in 20 fractions, should be reserved for patients with longer survival [36,37].
Recent technological advances have enabled the implementation of unconventional modalities such as three-dimensional conformal radiation therapy (3D-CRT), stereotactic body radiosurgery/radiotherapy (SBRS or SBRT), intensity-modulated radiation therapy-(IMRT), intraoperative radiation therapy (IORT) in the treatment of MASCC [48]. The rationale behind these advanced techniques of delivering radiation therapy is to maximise the dose to the neoplastic lesion (one or a few large fractions of 8 to 30 Gy per fraction under imaging guidance), while at the same time avoiding the surrounding normal cells with the goal to improve outcome while minimise morbidity [45,49].
Adverse events related to radiation therapy depend on the irradiated normal tissues and include bone marrow suppression, gastrointestinal toxicity, mucositis and even radiation-induced myelopathy which can become a chronic side-effect [5,17].
Recurrences in the irradiation field are especially important because up to 25% of patients already treated with RT, develop recurrent disease. The use of re-irradiation to the spine for recurrent MASCCs is recommended only if the risk of radiation myelopathy is low and the interval between RT courses exceeds 6 months [50,51]. Radiobiological data offer formulas for calculating this risk. SBRS has been applied to the setting of recurrence [45,52]. Nevertheless, the recommendations of the American Society for Radiation Oncology (ASTRO) are that patients with poor performance status should not be managed with SBRT [53]. Until recently, there is not enough evidence to support the superiority of SBRS over conventional fractionated radiation or decompressive surgery in this group of patients [54].
In the context of postoperative adjuvant treatment, radiation therapy can start 7 to 14 days after surgery using the default standard scheme of 30 Gy in 10 fractions in two weeks [5,15,55]. In patients with a good prognosis submitted to surgical treatment, highly conformal re-irradiation techniques must be considered to permit not only sufficient dose to the tumour, but also to spare the surrounding normal neural structures.
Key factors in deciding whether to pursue surgery before RT include spinal stability, presence of neurologic deficits, and patient prognosis [3]. Tables 2 and 3 summarise the general indications for surgery and radiation therapy, respectively.
Currently, the available literature supports the use of pre-irradiation surgical decompression in eligible surgical cancer patients with MASCCs [56]. Consequently, it is of extraordinary importance for each treatment modality (radiation alone vs surgical decompression followed by radiotherapy) to adequately select candidate patients to optimise the outcome and avoid unnecessary morbidity. For patients who meet surgical criteria, radiation therapy has an adjuvant role. Nonetheless, in patients unfit for surgery or where surgery would be inappropriate, radiotherapy should be the primary treatment [56].
Systemic therapy
In chemo-sensitive tumours (e.g., leukaemia, germ cell tumours, neuroblastoma, lymphomas), chemotherapy, either in combination with other treatments or as the primary modality, may have a role in the treatment of MASCCs [57–60]. The predominant advantage of using chemotherapy as the primary treatment of MASCCs in patients with chemo-sensitive tumours is the possibility to provide concurrent therapy to other possible foci of systemic disease as well as avoiding probable complications of surgery. In asymptomatic or with minimal symptoms MASCCs patients with lymphoma, chemotherapy as a neoadjuvant therapy is an attractive alternative reserving radiotherapy for patients non-responding to chemotherapeutic agents.
Currently, there is strong evidence that targeted therapy prolongs the survival of cancer patients with metastatic disease. Tyrosine kinase inhibitors (TKIs) have improved the median progressive-free survival and the treatment response rates of patients with anaplastic lymphoma kinase mutations or in tumours expressing epidermal growth factor receptor (EGFR) [61,62]. Moreover, immunotherapies are an additional therapeutic tool in a wide range of malignancies regarded as highly immunogenic [63–66].
Steroids
Corticosteroids are indicated for the initial treatment of MASCC. Loading intravenous (i.v.) dose is followed by a maintenance i.v. or per os dose. There is consensus that combination of steroids with RT is superior to RT alone [67]. Steroids have a beneficial effect in pain control and have direct cytotoxic effects on leukaemias and lymphomas [17,45]. An improvement of motor function following initiation of steroids is a positive prognostic factor, because it is associated with additional motor function recovery after definitive treatment [68]. Moreover, corticosteroids reduce spinal cord vasogenic oedema, thus protecting from the secondary complication of reduced arterial flow and subsequent ischaemia, infarction, and irreversible injury [45].
Steroid effectiveness is better exploited when administered immediately (i.e., ideally within 12-hours of symptom onset) once the diagnosis of MASCC is confirmed. Following definitive therapy with RT or surgery, a tapering schedule should be implemented to reduce the incidence of side effects. The optimal duration of steroid therapy is subject of debate. Toxicity from steroids has been demonstrated when usage exceeds 21 days and becomes more prominent at 40-days following initiation [67].
Numerous clinical trials have studied the role and dose of steroids [68–70]. Sorensen et al. performed a randomised single-blind trial, comparing two groups: one group with no steroid treatment and the other group that received a short course (13 days in total) of high-dose dexamethasone (96 mg iv loading dose, followed by 24 mg per os every six hours for three days and a ten-day tapering). The results demonstrated that 81% of patients in the corticosteroid group were ambulatory at three months vs 63% of patients in the non-steroid group (p = 0.0460), resulting in better functional outcome [68]. Extra caution should be applied to avoid using steroids in younger patients or in cases of undiagnosed primary cancer until a suitable diagnostic histological specimen is obtained, especially when malignant thymoma or lymphoma is suspected.
Supportive care and rehabilitation
Supportive therapy is a generic term under the umbrella of which are hosted physical therapy, management of bowel and/or bladder incontinence, prophylaxis of thromboembolic disease, adequate analgesia, access to specialist rehabilitation and finally psychological as well as social support.
Probably, pain management is the objective with the highest priority during the treatment pathway, due to its obvious and solid correlation, not only with the ability of patients to undergo rehabilitation but also with the quality of life. The potential aetiologies of pain are numerous, especially if surgery is performed. There are two principal types of pain and often occur in combination. Neuropathic pain (e.g., preexisting chemotherapy-related peripheral neuropathy, post-radiation or post-surgical fibrosis, radiculopathy from tumour invasion or from compression ab extrinseco), defined as “pain caused by a lesion or disease affecting the somatosensory system” [71,72]. It is the result of direct damage to the nervous system from a tumour or a metastatic lesion or from cancer treatment. It is estimated that 20% of cancer pain cases is purely neuropathic in origin. Neuropathic pain is typically chronic, and it manifests as recurrent, painful episodes or persisting continuously. Its consequences are spontaneous pain, increased pain sensitivity and loss of function. Nociceptive pain, on the other hand, (e.g., infection, postsurgical pain, failure of stabilisation, bone pain), is defined as “pain that arises from activation of nociceptors due to threatened or actual damage to non-neural tissue”. In contrast to neuropathic pain, in nociceptive pain, the somatosensory nervous system is functionally normal. Most of the times though, pain is mixed in nature thus neuropathic plus nociceptive components. Accurate diagnosis is of utmost importance for the choice of the best treatment scheme [73]. Steroids are effective in ameliorating inflammation, oedema and consequently pain, but the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and, more commonly, opiate, non-opiate or opioids medication are often required. These are administered per os, as transdermal therapeutic systems (TTS), as epidural or intrathecal pumps or as intravenous patient-controlled analgesia (PCA) for breakthrough pain. Use of specific molecules (e.g., pregabalin) for neuropathic pain found in medications (e.g., anxiolytics, tricyclic antidepressants, anticonvulsants) has also proven beneficial. Possible side-effects are cardiac arrhythmias, orthostatic hypotension, sedation and anticholinergic effects. As mentioned earlier, if NSAIDs and/or corticosteroids are used, gastric protection with PPIs should also be introduced. Nonpharmacologic therapies include transcutaneous electrical nerve stimulation (TENS), acupuncture, acupressure, cognitive therapies, psychology sessions, massage, yoga, assist devices (e.g. spinal braces), which can also help with pain control [74].
Stretching splints, passive stretching exercises and medications (e.g., benzodiazepines, baclofen, tizanidine) could be used to address a common complication of upper motor neuron lesion which is spasticity.
Decreased intestinal motility and constipation is another frequent symptom of multifactorial origin e.g., a side effect from the use of analgesics, decreased physical activity, autonomic dysfunction (i.e. upper motor neuron lesions that cause sphincter hyperreflexia and retention). Treatment is conservative with combined use of stool softeners and oral osmotic laxatives (e.g. polyethylene glycol). Oral colonic stimulants of peristalsis (e.g. senna) are also used to promote normal bowel movements and avoid exacerbation of pain secondary to Valsalva maneuvers. Patients with more severe constipation may require lactulose, enema, or suppository. On the other hand, lesions of the lower motor neuron cause fecal incontinence. Bulk forming agents (e.g. psyllium) or manual evacuation schedules are used.
Intermittent catheterisation and anticholinergic agents can be implemented for upper motor neuron lesions which cause external sphincter hyperreflexia and urinary retention, whereas indwelling catheters and bladder retraining can be applied for lower motor neuron bladder patterns manifesting with sphincter flaccidity and incontinence.
Cancer patients have a 4-to-7-fold higher risk of venous thromboembolism (VTE) (Deep Venous Thrombosis-DVT and Pulmonary Embolism-PE), compared to healthy individuals [75]. If no contraindications are present, application of graduated elastic compression stockings, physiotherapy with leg exercises, and use of prophylaxis with anticoagulants (i.e., Direct Oral AntiCoagulants-DOACs, Low-Molecular-Weight Heparins -LMWHs) are recommended [75]. The duration of thromboprophylactic treatment is based on the presence of ongoing risk factors, comorbidities, overall clinical condition and return to mobility. Many of the patients with MASCC, because of their low performance status and decreased mobility due to accompanying risk factors (e.g. impaired state of consciousness, pain, pathological fractures, cachexia), are confined in bed and consequently, should be nursed in bed. Spinal precautions (e.g. postural braces) ensuring the stability of the vertebral column and management of decubitus ulcers, are equally important goals of patient’s treatment [76].
CONCLUSIONS AND FUTURE DIRECTIONS
MASCC is a relatively frequent oncological emergency. Optimal management requires close cooperation from a multidisciplinary team consisting of radiologists, oncologists, neurosurgeons, nurses and physiotherapists for an immediate and accurate diagnosis, the implementation of the most effective treatment, management of complications and comorbidities. Because of the urgency of such a situation in high-risk patients, clinicians should be aware of the signs, symptoms and how to address them in case of development. Initial surgical decompression in eligible surgical candidates with MASCCs has a prominent role in the treatment. In patients unfit for surgery or where surgery would be inappropriate, radiation therapy should be the primary treatment. Evolution of minimally invasive surgical techniques and application of modern radiation therapy techniques should be further explored. Chemotherapy seems to play a role in carefully selected patients with tumours sensitive to pharmacologic agents, especially in combination with other treatments. Bisphosphonates have demonstrated a role in the reduction of the number of skeletal complications, metastatic bone pain, treatment of hypercalcaemia and improvement of quality of life. Steroids have a beneficial role in oedema and pain control improving the clinical outcome. Finally, supportive therapies of care and rehabilitation through a network-led service for the delivery of services are of great significance to promoting the quality of life of patients with MASCC.
Future research on this clinical entity should focus on two main pathways: First, to develop diagnostic algorithms leading physicians to the proper selection of appropriate imaging techniques and second, to apply guidelines which can be used by the multidisciplinary team for the careful choice of the suitable treatment modality. Bearing always in mind that the therapeutic goal for this group of patients is to improve or maintain the quality of life in these patients.
Conflict of interest disclosure: None to declare
Declaration of funding sources: None to declare
Author contributions: SA, conception and design; IH, RA, DS, analysis and interpretation of the data; IH, DK, drafting of the article; DS, DK, SA, critical revision of the article for important intellectual content; all authors, final approval of the article.
REFERENCES
- Hershkovich O, Sakhnini M, Gara S, Caspi I, Lotan R. Acute Metastatic Spinal Cord Compression: Urgent Surgery versus Radiotherapy and Treatment Result Prediction versus Actual Results. Curr Oncol Tor Ont. 2022;29(10):7420–29.
- Lawton AJ, Lee KA, Cheville AL, Ferrone ML, Rades D, Balboni TA, et al. Assessment and Management of Patients With Metastatic Spinal Cord Compression: A Multidisciplinary Review. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(1):61–71.
- Quraishi NA, Esler C. Metastatic spinal cord compression. BMJ [Internet]. [cited 2011 Apr 27];342:d2402. Available from: https://www.bmj.com/content/342/bmj.d2402.full.
- Boussios S, Cooke D, Hayward C, Kanellos FS, Tsiouris AK, Chatziantoniou AA, et al. Metastatic Spinal Cord Compression: Unraveling the Diagnostic and Therapeutic Challenges. Anticancer Res. 2018;38(9):4987–97.
- Schiff D. Spinal cord compression. Neurol Clin. 2003;21(1):67–86.
- Levack P, Graham J, Collie D, Grant R, Kidd J, Kunkler I, et al. Don’t wait for a sensory level–listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol R Coll Radiol G B. 2002;14(6):472–80.
- Perrin RG, Laperriere NJ, Loblaw DA, Laxton AW. Spinal axis metastases. Cancer metastatic to the central nervous system. [Internet]. 2002 Winter [cited 2023 Mar 19];14:341-61. Available from: https://www.soc-neuro-onc.org/UploadedFiles/Levin/Levin_ch14_p341-361.pdf
- Klimo P, Thompson CJ, Kestle JRW, Schmidt MH. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro-Oncol. 2005;7(1):64–76.
- Kwok Y, Regine WF, Patchell RA. Radiation therapy alone for spinal cord compression: time to improve upon a relatively ineffective status quo. J Clin Oncol. 2005;23(15):3308–10.
- Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol R Coll Radiol G B. 2003;15(4):211–17.
- Perrin RG, Laxton AW. Metastatic spine disease: epidemiology, pathophysiology, and evaluation of patients. Neurosurg Clin N Am. 2004;15(4):365–73.
- Quinn JA, DeAngelis LM. Neurologic emergencies in the cancer patient. Semin Oncol. 2000;27(3):311–21.
- Abrahm JL. Assessment and treatment of patients with malignant spinal cord compression. J Support Oncol. 2004;2(5):377–88, 391; discussion 391-393, 398, 401.
- Sciubba DM, Gokaslan ZL. Diagnosis and management of metastatic spine disease. Surg Oncol. 2006;15(3):141–51.
- Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet Lond Engl. 2005;366(9486):643–48.
- Fattal C, Fabbro M, Gelis A, Bauchet L. Metastatic paraplegia and vital prognosis: perspectives and limitations for rehabilitation care. Part 1. Arch Phys Med Rehabil. 2011;92(1):125–33.
- Hammack JE. Spinal cord disease in patients with cancer. Contin Minneap Minn. 2012;18(2):312–27.
- Penas-Prado M, Loghin ME. Spinal cord compression in cancer patients: review of diagnosis and treatment. Curr Oncol Rep. 2008;10(1):78–85.
- Sciubba DM, Petteys RJ, Dekutoski MB, Fisher CG, Fehlings MG, Ondra SL, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine. 2010;13(1):94–108.
- Harel R, Angelov L. Spine metastases: current treatments and future directions. Eur J Cancer Oxf Engl 1990. 2010; 46(15):2696–707.
- Sun H, Nemecek AN. Optimal management of malignant epidural spinal cord compression. Hematol Oncol Clin North Am. 2010;24(3):537–51.
- Rades D, Abrahm JL. The role of radiotherapy for metastatic epidural spinal cord compression. Nat Rev Clin Oncol. 2010;7(10):590–98.
- Kuris EO, McDonald CL, Palumbo MA, Daniels AH. Evaluation and Management of Cauda Equina Syndrome. Am J Med. 2021;134(12):1483–89.
- McCurdy MT, Shanholtz CB. Oncologic emergencies. Crit Care Med. 2012;40(7):2212–22.
- Venkitaraman R, Sohaib SA, Barbachano Y, Parker CC, Khoo V, Huddart RA, et al. Detection of occult spinal cord compression with magnetic resonance imaging of the spine. Clin Oncol R Coll Radiol G B. 2007;19(7):528–31.
- Huang J, Jatoi A. Morbidity and mortality in patients with cancer who become nonambulatory after spinal cord compression: a case series on end-of-life care. J Palliat Med. 2009;12(3):219–22.
- Helweg-Larsen S, Sørensen PS, Kreiner S. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46(5):1163–69.
- Robson P. Metastatic spinal cord compression: a rare but important complication of cancer. Clin Med Lond Engl. 2014;14(5):542–45.
- Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30(19):2186–91.
- Murtaza H, Sullivan CW. Classifications in Brief: The Spinal Instability Neoplastic Score. Clin Orthop Relat Res. 2019;477(12):2798–803.
- Roberts TT, Leonard GR, Cepela DJ. Classifications In Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin Orthop Relat Res. 2017;475(5):1499–504.
- Kirshblum S, Snider B, Rupp R, Schmidt M. Updates of the International Standards for Neurologic Classification of Spinal Cord Injury: 2015 and 2019. Phys Med Rehabil Clin N Am. 2020;31(3):319-30.
- Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol. 2008;7(5):459–66.
- Raj VS, Lofton L. Rehabilitation and treatment of spinal cord tumors. J Spinal Cord Med. 2013;36(1):4–11.
- Witham TF, Khavkin YA, Gallia GL, Wolinsky J-P, Gokaslan ZL. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol. 2006;2(2):87–94.
- Ropper AE, Ropper AH. Acute Spinal Cord Compression. N Engl J Med. 2017;376(14):1358–69.
- Maranzano E, Bellavita R, Rossi R, De Angelis V, Frattegiani A, Bagnoli R, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(15):3358–65.
- Schirmer CM, Malek AM, Kwan ES, Hoit DA, Weller SJ. Preoperative embolization of hypervascular spinal metastases using percutaneous direct injection with n-butyl cyanoacrylate: technical case report. Neurosurgery. 2006;59(2):e431-32.
- Guzman R, Dubach-Schwizer S, Heini P, Lovblad K-O, Kalbermatten D, Schroth G, et al. Preoperative transarterial embolization of vertebral metastases. Eur Spine J. 2005;14(3):263–68.
- Fourney DR, Schomer DF, Nader R, Chlan-Fourney J, Suki D, Ahrar K, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(1): Suppl:21–30.
- Sadeghi-Naini M, Aarabi S, Shokraneh F, Janani L, Vaccaro AR, Rahimi-Movaghar V. Vertebroplasty and Kyphoplasty for Metastatic Spinal Lesions: A Systematic Review. Clin Spine Surg. 2018;31(5):203–10.
- Health Quality Ontario. Vertebral Augmentation Involving Vertebroplasty or Kyphoplasty for Cancer-Related Vertebral Compression Fractures: A Systematic Review. Ont Health Technol Assess Ser. 2016;16(11):1–202.
- Rades D, Al-Salool A, Staackmann C, Cremers F, Cacicedo J, Lomidze D, et al. A New Clinical Instrument for Estimating the Ambulatory Status after Irradiation for Malignant Spinal Cord Compression. Cancers. 2022;14(15):3827.
- Choi D, Crockard A, Bunger C, Harms J, Kawahara N, Mazel C, et al. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J. 2010;19(2):215–22.
- Ribas ESC, Schiff D. Spinal cord compression. Curr Treat Options Neurol. 2012;14(4):391–401.
- Rades D, Douglas S, Veninga T, Stalpers LJA, Hoskin PJ, Bajrovic A, et al. Validation and simplification of a score predicting survival in patients irradiated for metastatic spinal cord compression. Cancer. 2010;116(15):3670–73.
- Rades D, Lange M, Veninga T, Stalpers LJA, Bajrovic A, Adamietz IA, et al. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2011;79(2):524–30.
- Lo SS, Sahgal A, Hartsell WF, Lutz ST, Kardamakis D, van der Linden Y, et al. The treatment of bone metastasis with highly conformal radiation therapy: a brave new world or a costly mistake? Clin Oncol R Coll Radiol G B. 2009;21(9):662–64.
- Lo SS, Chang EL, Yamada Y, Sloan AE, Suh JH, Mendel E. Stereotactic radiosurgery and radiation therapy for spinal tumors. Expert Rev Neurother. 2007;7(1):85–93.
- Nieder C, Grosu AL, Andratschke NH, Molls M. Proposal of human spinal cord reirradiation dose based on collection of data from 40 patients. Int J Radiat Oncol Biol Phys. 2005;61(3):851–55.
- Sahgal A, Ma L, Weinberg V, Gibbs IC, Chao S, Chang U-K, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(1):107–16.
- Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6(1):15–24.
- Hashmi A, Guckenberger M, Kersh R, Gerszten PC, Mantel F, Grills IS, et al. Re-irradiation stereotactic body radiotherapy for spinal metastases: a multi-institutional outcome analysis. J Neurosurg Spine. 2016;25(5):646–53.
- Bate BG, Khan NR, Kimball BY, Gabrick K, Weaver J. Stereotactic radiosurgery for spinal metastases with or without separation surgery. J Neurosurg Spine. 2015;22(4):409–15.
- De Felice F, Piccioli A, Musio D, Tombolini V. The role of radiation therapy in bone metastases management. Oncotarget. 2017;8(15):25691–99.
- Rades D, Küchler J, Graumüller L, Abusamha A, Schild SE, Gliemroth J. Radiotherapy with or without Decompressive Surgery for Metastatic Spinal Cord Compression: A Retrospective Matched-Pair Study Including Data from Prospectively Evaluated Patients. Cancers. 2022;14(5):1260.
- Chamberlain MC. Leukemia and the nervous system. Curr Oncol Rep. 2005;7(1):66–73.
- Gale J, Mead GM, Simmonds PD. Management of spinal cord and cauda equina compression secondary to epidural metastatic disease in adults with malignant germ cell tumours. Clin Oncol R Coll Radiol G B. 2002;14(6):481–90.
- De Bernardi B null, Pianca C, Pistamiglio P, Veneselli E, Viscardi E, Pession A, et al. Neuroblastoma with symptomatic spinal cord compression at diagnosis: treatment and results with 76 cases. J Clin Oncol. 2001;19(1):183–90.
- Matsubara H, Watanabe K, Sakai H, Chang H, Fujino H, Higashi Y, et al. Rapid improvement of paraplegia caused by epidural involvements of Burkitt’s lymphoma with chemotherapy. Spine. 2004;29(1):e4-6.
- Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, Dundar Y, et al. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev. 2021;3(3):CD010383.
- Solomon BJ, Mok T, Kim D-W, Wu Y-L, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77.
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–13.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627–39.
- Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–18.
- Ugurel S, Röhmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A, et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies. Eur J Cancer. 2016;53:125–34.
- Kumar A, Weber MH, Gokaslan Z, Wolinsky J-P, Schmidt M, Rhines L, et al. Metastatic Spinal Cord Compression and Steroid Treatment: A Systematic Review. Clin Spine Surg. 2017;30(4):156–63.
- Sørensen S, Helweg-Larsen S, Mouridsen H, Hansen HH. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer. 1994;30a(1):22–7.
- Skeoch GD, Tobin MK, Khan S, Linninger AA, Mehta AI. Corticosteroid Treatment for Metastatic Spinal Cord Compression: A Review. Glob Spine J. 2017;7(3):272–9.
- Maranzano E, Latini P, Beneventi S, Perruci E, Panizza BM, Aristei C, et al. Radiotherapy without steroids in selected metastatic spinal cord compression patients. A phase II trial. Am J Clin Oncol. 1996;19(2):179–83.
- Edwards HL, Mulvey MR, Bennett MI. Cancer-Related Neuropathic Pain. Cancers. 2019;11(3):373.
- Yoon SY, Oh J. Neuropathic cancer pain: prevalence, pathophysiology, and management. Korean J Intern Med. 2018; 33(6):1058–69.
- Stubblefield MD, Bilsky MH. Barriers to rehabilitation of the neurosurgical spine cancer patient. J Surg Oncol. 2007;95(5):419–26.
- Coutaux A. Non-pharmacological treatments for pain relief: TENS and acupuncture. Joint Bone Spine. 2017;84(6):657–61.
- Gervaso L, Dave H, Khorana AA. Venous and Arterial Thromboembolism in Patients With Cancer: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncology. 2021;3(2):173–90.
- Ferris A, Price A, Harding K. Pressure ulcers in patients receiving palliative care: A systematic review. Palliat Med. 2019;33(7):770–82.