ACHAIKI IATRIKI | 2022; 41(3):135-145
Review
Nikolaos Papathanasiou1, Konstantinos Papadimitropoulos1, Maria Spiliotopoulou1, Eleni Karagkouni2, Dimitrios Apostolopoulos1
1Nuclear Medicine & PET/CT Department, University Hospital of Patras, Rio, Patras, Greece
2Radiology Department, University Hospital of Patras, Rio, Patras, Greece
Received: 28 Jan 2022; Accepted: 15 Apr 2022
Corresponding author: Nikolaos Papathanasiou, Nuclear Medicine & PET/CT Department, University Hospital of Patras, Rio, Patras 26504, Greece, Tel.: +30 6978 774871, E-mail: nikopapath@googlemail.com
Key words: Positron emission tomography (PET), PET/CT, hybrid imaging, cancer, oncology
Abstract
PET/CT is a new generation, hybrid, whole-body imaging modality, which combines the functional imaging of cellular metabolism with Positron Emission Tomography (PET) plus the detailed depiction of human anatomy with Computed Tomography (CT). Nowadays, it is an established modality with approved clinical indications in oncology, while it has been incorporated in evidence-based diagnostic algorithms and guidelines. By reviewing recent literature, the current paper illustrates, concisely, the main strengths, limitations, and whole spectrum of clinical applications of PET/CT in the management of oncological patients.
INTRODUCTION
PET/CT is a state-of-the art, hybrid, whole-body imaging modality, which combines two methods in a single session: the functional tracing of cellular metabolism with Positron Emission Tomography (PET) plus the detailed, high-resolution depiction of human anatomy with Computed Tomography (CT). Since its initial experimental introduction in the 90’s, PET/CT has considerably evolved and been implemented into routine oncological practice. Nowadays, it is an established modality with miscellaneous clinical indications, while it has been incorporated into various evidence-based algorithms in Oncology [1]. PET/CT is no longer regarded as a “luxury”’ but as a mainstay of routine clinical practice affecting treatment decisions in oncological patients.
More than 90-95% of PET/CT studies are performed with the use of the radioactive tracer 18Fluoro-Deoxy-Glucose (FDG). FDG is a non-specific tracer, yet it has proven extremely efficient in clinical practice. It is a glucose analogue labelled with radioactive 18F, which emits positrons. FDG is injected intravenously, rapidly distributes throughout the body and shows avid uptake by cancerous cells (Figure 1). Malignant cells have increased metabolic needs, which are fulfilled by anaerobic glycolysis; hence, these cells show avid uptake of glucose and glucose analogues like FDG. Within the cancer cell, FDG is not metabolized, but it continuously accumulates, being trapped into the cytoplasm.
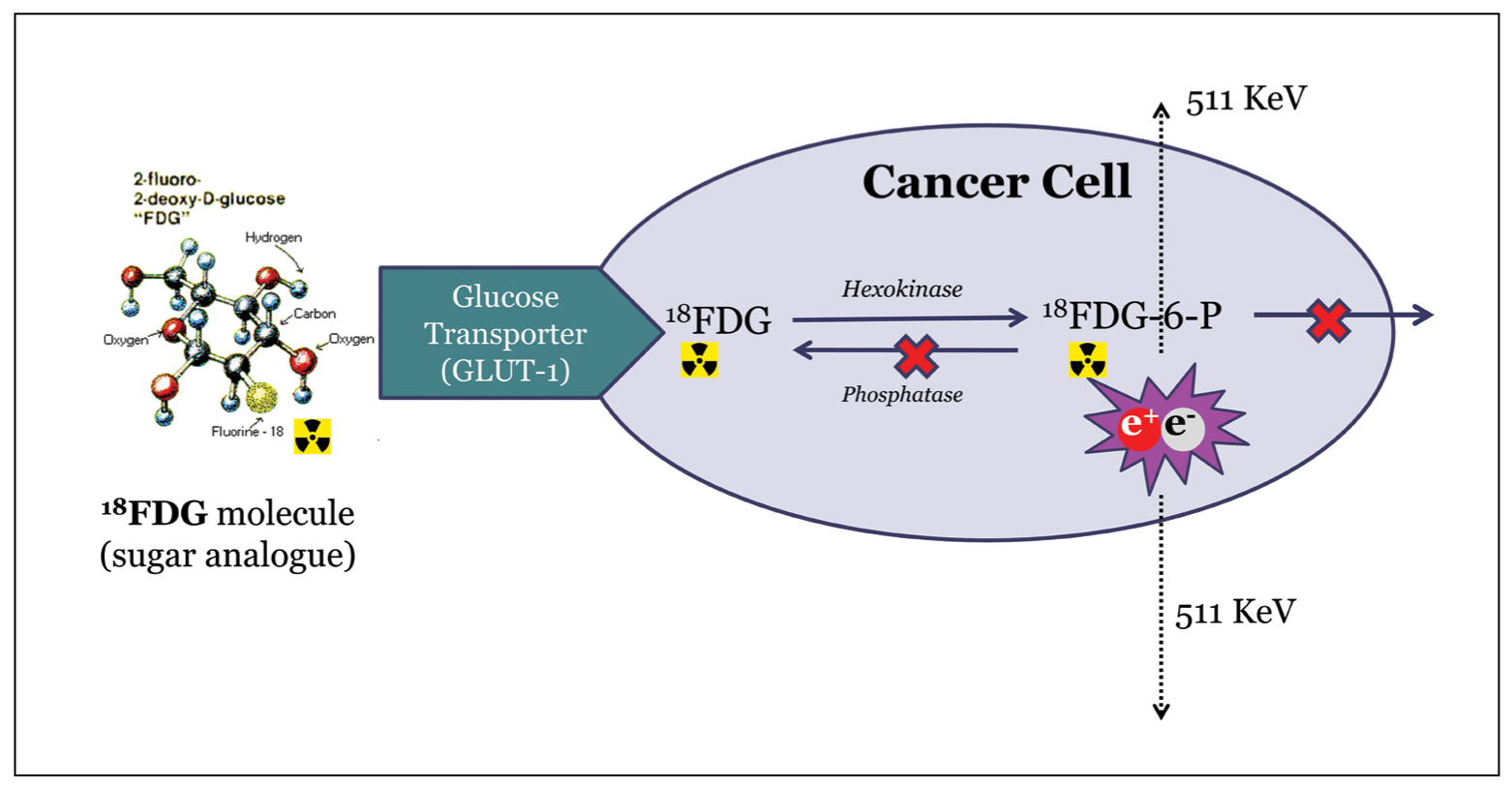 Figure 1. FDG uptake by the malignant cell. 18Fluoro-Deoxy-Glucose (FDG) is avidly taken up by the cancer cell. This transport is facilitated by the Glucose Transporter enzymes (GLUT). Inside the cell, FDG is phosphorylated via the hexokinase, but, unlike normal glucose, it does not enter the Krebs cycle and does not undergo further metabolism. Thus, FDG is continuously trapped inside the cytoplasm, and positrons are emitted by the radioactive 18F. The positrons annihilate with human body electrons and two gamma-ray photons with energies of 511 KeV are emitted in the opposite directions. The photons penetrate the human body and are detected by PET crystals of the scanner.
Figure 1. FDG uptake by the malignant cell. 18Fluoro-Deoxy-Glucose (FDG) is avidly taken up by the cancer cell. This transport is facilitated by the Glucose Transporter enzymes (GLUT). Inside the cell, FDG is phosphorylated via the hexokinase, but, unlike normal glucose, it does not enter the Krebs cycle and does not undergo further metabolism. Thus, FDG is continuously trapped inside the cytoplasm, and positrons are emitted by the radioactive 18F. The positrons annihilate with human body electrons and two gamma-ray photons with energies of 511 KeV are emitted in the opposite directions. The photons penetrate the human body and are detected by PET crystals of the scanner.
FDG-PET/CT imaging is performed in two stages. First, CT is performed for attenuation correction of the PET images and anatomical localization. Then, PET is conducted detecting the photons emitted from FDG. Both PET and CT images are finally combined together into fusion images [2,3]. Fusion provides us with valuable information regarding the size, morphology, location, and extent of malignant lesions plus their metabolic activity. Fused PET/CT exhibits inherent advantages in oncological imaging:
It shows high sensitivity for the detection of malignant lesions even in regions with normal anatomy; hence, PET/CT implementation may result in early cancer detection.
It detects neoplastic lesions in regions, not easily evaluated by conventional anatomical imaging methods (CT/MRI) due to distorted anatomy after previous surgery, radiotherapy or other treatments.
Post treatment, it may accurately distinguish between metabolically active, viable neoplastic tissue and non-viable, necrotic or fibrotic residual tissue.
PET/CT achieves high diagnostic accuracy, which translates into significant changes of the therapeutic strategy in almost 30% of oncological patients. The major clinical indications of PET/CT are summarized into: a) initial staging of neoplastic disease, b) evaluation of treatment response and c) cancer restaging plus timely and accurate detection of disease recurrence [1] (Table 1).
The main limitations of FDG-PET are the false positive findings in cases of active inflammation. FDG is a non-specific tracer and may be taken up by macrophages and lymphocytes, which accumulate in inflammatory regions. As a result, various inflammatory entities may act as sources of false positive findings such as sarcoid, tuberculosis and other infections, abscesses, abdomino-pelvic inflammations, and post-traumatic/post-surgical changes. There are also benign tumors and other entities showing false-positive, increased FDG uptake including Warthin tumors, benign bone tumors, inflammatory pseudo-tumor, thyroid adenomas, and uterine fibroids.
On the flip side of PET-positive inflammation, there are neoplasms with slow metabolic rate, low glucose metabolism, thus low FDG uptake: differentiated thyroid cancer and prostate cancer with some exceptions, indolent lymphomas, low-grade sarcomas and neuro-endocrine tumors (NETs) and some mucinous carcinomas. These may result in false negative findings.
Reporting of PET/CT studies is mainly based on visual image interpretation. The nuclear medicine physician evaluates the intensity of FDG uptake by suspicious target lesions and compares this uptake with adjacent background FDG activity in order to, finally, decide whether the lesion is abnormal/malignant. To improve this subjective, reporter-dependent approach, quantification of FDG uptake is applied by means of a numeric ratio, Standardized Uptake Value, SUV=Activity in a specified region of interest/ Total injected Activity normalized by body weight. Higher SUV values correspond to higher probabilities of malignancy; however, they cannot, alone, substitute for visual, qualitative interpretation. SUV values show considerable overlap between malignant and benign-inflammatory lesions with no absolute cut-off thresholds being specific for malignancy. Moreover, SUV calculations show significant variability dependent on multiple factors including body habitus, blood glucose levels, uptake time, type of PET/CT scanner and software, image reconstruction algorithms, timing of treatment and size of the lesion.
Clinical Applications
PET/CT is the imaging method of choice in the initial staging of the majority of lymphomas, with the most frequent types of Hodgkin’s, Diffuse Large B cell and follicular lymphomas showing increased FDG uptake [4]. PET/CT brings increased sensitivity in the detection of lymphomatous nodal disease even in small/normal-sized nodes. It is characterized by higher sensitivity than CT in the detection of extra-nodal disease, specifically in the spleen and bone marrow. PET/CT findings lead to upstaging in up to 25% of Hodgkin lymphomas, and this translates into intensified therapy. It has excellent Negative Predictive Value (NPV>95%) in the detection of bone marrow involvement in Hodgkin’s lymphoma [5], essentially replacing bone marrow biopsy [6,7]: a negative PET rules out bone marrow disease in Hodgkin’s patients. PET/CT is superior to other imaging methods in the initial staging of aggressive non-Hodgkin lymphomas detecting occult disease in sites not previously suspected [8].
Apart from staging, PET/CT is applied in the early therapeutic evaluation of Hodgkin’s lymphoma in the form of interim PET/CT performed after 2-3 initial cycles of chemotherapy [9]. Patients with negative interim PET and no hypermetabolic lesions identified may continue with the same effective treatment or switch to less aggressive, less toxic protocols. On the contrary, patients who do not show PET response may be subjected to more aggressive treatment in order to eradicate hypermetabolic active disease. Randomized controlled trials have proven that interim PET/CT shows high NPV for final treatment response and for increased progression free survival in Hodgkin’s lymphoma [10-12]. Recently, the accuracy in reporting and interpretating interim and post-treatment PET/CT studies has increased by applying specific objective criteria: Deauville 5-scale criteria [13]. High Deauville uptake score of 4-5, in case FDG uptake in lesions exceeds liver activity, corresponds to active neoplastic disease. FDG uptake in lesions, which is equal or lower than in the mediastinal blood-pool, is interpreted as negative for disease: Deauville score of 1-2.
PET/CT is the imaging method of choice for final post-treatment assessment of lymphomas showing excellent NPV and superior diagnostic accuracy compared with CT [14]. After treatment, a significant proportion of patients show residual anatomic lesions on CT; yet, in only a small minority of cases, these lesions correspond to residual disease. PET/CT has high diagnostic accuracy in the evaluation of residual tissue and may distinguish between PET-negative fibrotic/necrotic tissue and PET-positive, active residual disease (Figure 2). The modality also has high NPV in the evaluation of megatherapy before stem cell transplantation. In this setting, a favorable PET response is associated with better progression free survival and overall survival [15].
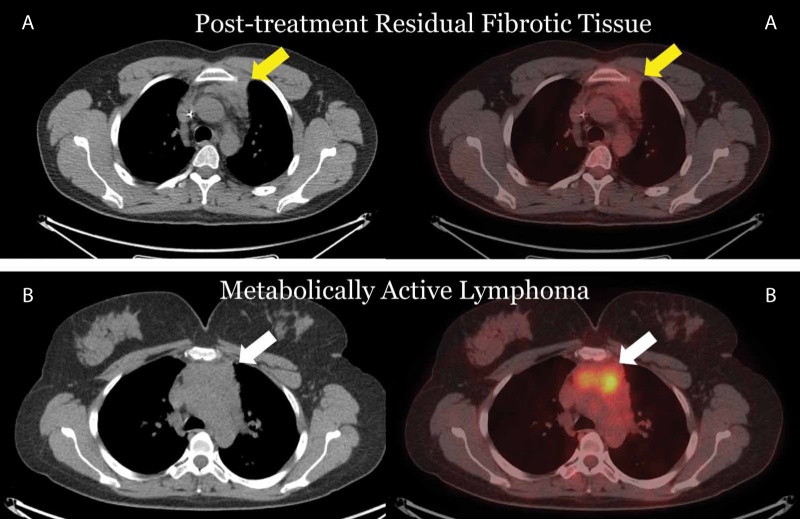 Figure 2. Hodgkin’s Lymphomas post treatment. A. Treatment response with residual anterior mediastinal mass not showing increased FDG uptake (yellow arrow). B. Case of residual active disease with lobulated mediastinal soft-tissue mass showing increased metabolic activity (white arrow).
Figure 2. Hodgkin’s Lymphomas post treatment. A. Treatment response with residual anterior mediastinal mass not showing increased FDG uptake (yellow arrow). B. Case of residual active disease with lobulated mediastinal soft-tissue mass showing increased metabolic activity (white arrow).
PET/CT has valid clinical roles and several applications in the evaluation and management of lung cancer. In the assessment of solid pulmonary nodules of sufficient size (≥8-10 mm), it shows high sensitivity (~90%) in detecting lung cancer. The specificity is somewhat lower (~80-85%) due to inflammatory, false positive nodules in cases of sarcoid, tuberculosis or other granulomatous disease and infections [16,17]. Pulmonary carcinoids and adenocarcinomas in situ may be false negative on PET, especially when the latter appear as pure ground-glass or semi-solid nodules [18].
PET/CT brings improvements in the accurate staging of lung cancer and optimizes therapeutic decisions [19]. It shows superior diagnostic accuracy compared with CT in the evaluation of mediastinal nodes with sensitivity >80% and specificity >90%. PET helps in overcoming the limitations of CT, the latter relying on size (short axis >1 cm) and morphological criteria: small and normal-sized nodes may harbor neoplastic disease, while slightly enlarged nodes may be reactive, not associated with malignancy. PET can, however, miss occult mediastinal disease, especially in large central tumors [20,21]. Therefore, it cannot replace minimally invasive methods of nodal sampling in all patients, but rather guide sampling to specific suspicious nodal stations.
PET/CT is the modality of choice for the detection of distal metastases with sensitivity and specificity ≥ 90%. Almost 20-35% of newly diagnosed lung cancers are already metastatic, with 40% of these patients having skeletal metastases (Figure 3). PET can detect hypermetabolic bone lesions in early stage before inducing anatomical skeletal changes such as bone destruction-lysis or sclerosis. The modality may distinguish between adrenal metastases and benign adenomas with high accuracy. It detects metastases in unsuspected regions such as soft-tissue deposits and hypermetabolic subcutaneous nodules. PET/CT implementation leads to changes in clinical staging in a significant proportion of patients (25-60%), thus resulting in corresponding treatment modifications: avoidance of futile thoracotomies, accurate definition of treatment volumes in radiotherapy planning, change of therapeutic plan from a curative to a palliate approach etc.
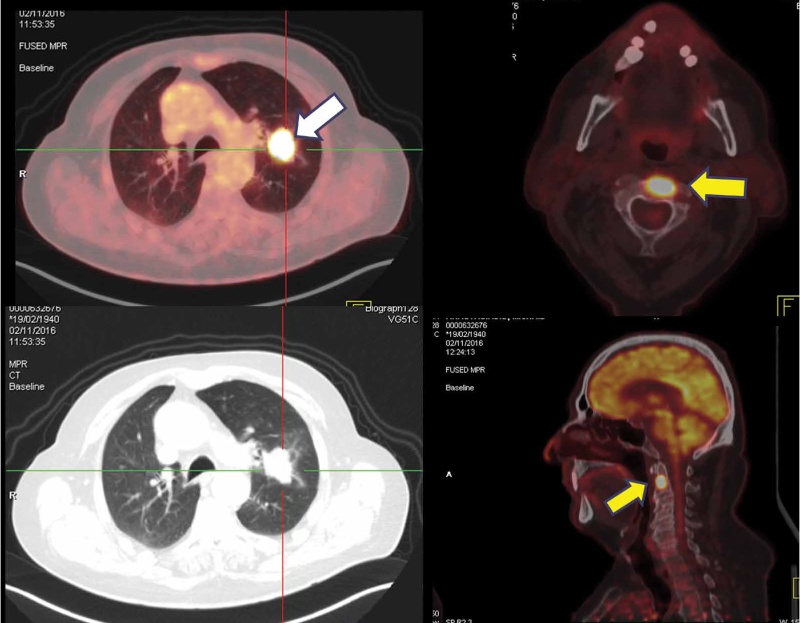 Figure 3. Metastatic lung cancer. Hypermetabolic left lung cancer mass (white arrow) with a solitary FDG-avid metastatic bone lesion in C2 vertebra (yellow arrow).
Figure 3. Metastatic lung cancer. Hypermetabolic left lung cancer mass (white arrow) with a solitary FDG-avid metastatic bone lesion in C2 vertebra (yellow arrow).
In the evaluation of treatment response, PET/CT is increasingly being applied as it shows inherent advantages: it may distinguish between viable tumor and post-treatment changes, it evaluates the whole tumor burden and may reveal metabolic treatment response irrespective of anatomical changes which may underestimate treatment effect. In restaging, PET/CT is applied as a problem-solving tool to detect residual or recurrent disease, especially when there are equivocal or difficult to interpret findings by other imaging methods.
PET/CT is not typically indicated for the initial diagnosis and detection of primary head-neck squamous cell carcinomas with the exception of unknown primary cancer presenting with cervical nodal metastases [22-24]. The diagnostic detection yield, in this setting, is around 25-55%. Most frequently, the primary tumor resides in the palatine or lingual tonsils or in the tongue base. The detection of the primary tumor has critical prognostic and therapeutic implications, since it can guide surgical planning, mode of neck dissection or definition of radiotherapy volumes [25]. PET/CT has superior diagnostic accuracy compared with other modalities for the evaluation of disease-involved nodes in head-neck cancer [22,26] (Figure 4). It is the modality of choice for the detection of distal metastatic disease and other synchronous tumors [24,27]. It is indicated by oncological guidelines for the accurate evaluation of post-treatment neck, after radical chemo-radiation therapy [22]. It is highly accurate in the assessment of post-treatment neck, which is hindered by extensive post-surgical changes including flap reconstructions and neck dissections, distortion of normal anatomy, oedema, asymmetries, and obliteration of fat planes. PET/CT has high NPV post radical chemo-radiation therapy sparing these patients from unnecessary morbid nodal dissections [28,29]. In the differentiated thyroid cancer, PET/CT has only one major clinical application: the evaluation of patients with increased Thyroglobulin levels and negative whole-body iodine scans indicating disease de-differentiation and more aggressive clinical behavior [30].
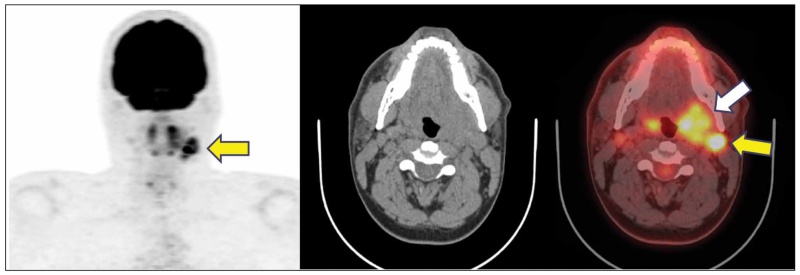 Figure 4. Oropharyngeal cancer. Case of oropharyngeal cancer with hypermetabolic enlargement of the left palatine tonsil (white arrow) obliterating the adjacent parapharyngeal space. There are FDG-avid, disease-involved ipsilateral jugular nodes (yellow arrow). Oropharyngeal cancer is the most common head-neck cancer in the western world, often arising in the tongue base or in the palatine tonsils. It, often, shows a predictable pattern of nodal spread from superior to inferior, first involving the upper jugular chain and then the middle and lower nodes.
Figure 4. Oropharyngeal cancer. Case of oropharyngeal cancer with hypermetabolic enlargement of the left palatine tonsil (white arrow) obliterating the adjacent parapharyngeal space. There are FDG-avid, disease-involved ipsilateral jugular nodes (yellow arrow). Oropharyngeal cancer is the most common head-neck cancer in the western world, often arising in the tongue base or in the palatine tonsils. It, often, shows a predictable pattern of nodal spread from superior to inferior, first involving the upper jugular chain and then the middle and lower nodes.
PET/CT is not routinely indicated in early stage I-II melanomas offering no significant diagnostic or prognostic information [31]. The method has unacceptably low sensitivity (30-50%) in the determination of regional lymph node status, since it cannot detect metastatic burden in small nodes <5-10 mm; hence, it cannot substitute for sentinel node biopsy, which remains the clinical standard of care in the evaluation of locoregional nodal status [32]. The diagnostic benefit of PET/CT implementation increases as the clinical stage increases. In melanomas with high Breslow thickness (>4,0 mm), the modality can detect disease-involved regional lymph nodes and additional distal metastases in almost 30% of patients affecting subsequent treatment strategy. PET/CT is indicated by clinical guidelines in stage III-IV melanomas and has been included in the corresponding algorithms. It also appears as a promising tool in the evaluation of patients treated with targeted therapy and immunotherapy as it may verify metabolic treatment response irrespective of anatomical changes [33,34]. The assessment of immunotherapy response is challenging, since, unlike conventional chemotherapy, the neoplastic lesions may, initially, enlarge before shrinking or the final response may occur despite the presence of new lesions; thus, new appropriate criteria for response evaluation are needed. PET/CT is also the modality of choice for detecting distal metastases in the staging and restaging of melanoma patients [31].
PET/CT is not a proper modality for the initial diagnosis and T-staging of oesophageal cancer because it misses small superficial tumors and cannot, accurately, determine the exact depth of tumor penetration through the eosophageal wall. However, PET/CT may depict advanced T-stage tumors (T3-T4) with the CT component of the study showing stranding of the adjacent peri-oesophageal fat, obliteration of fat planes and displacement or indentation of the mediastinum or other structures [35]. The modality has low sensitivity in the detection of regional nodes, which may be obscured by FDG activity in the adjacent, hypermetabolic primary tumor, yet it shows potential to detect distal nodes in the mediastinum, abdomen or supra-clavicular regions [35-37] (Figure 5). Guidelines appreciate the strengths of PET/CT to detect distant metastases with high specificity and metastatic lesions not identifiable by other methods. By doing this, it affects treatment decisions and selects patients suitable for radical treatment. Clinical guidelines suggest PET/CT for the evaluation of treatment response after pre-operative or radical chemoradiation. In gastric cancer, the primary tumor evaluation is hampered by low FDG uptake in certain histologic types such as mucinous, signet-ring and diffuse cancers and by incidental normal or inflammatory FDG uptake in the gastric wall. PET nodal staging shares the same properties as in oesophageal cancer. The method is not accurate in the evaluation of peri-gastric nodes, yet it may detect distal nodes outside of the typical lymphadenectomy bed, thus altering treatment plan [35]. It is also highly accurate in the detection of distant metastases in the liver or peritoneum. Gastric cancer often gives metastases in the peritoneum appearing as hypermetabolic soft-tissue nodules, peritoneal plaques or diffuse infiltrating stranding in the omentum, mesentery or other peritoneal spaces.
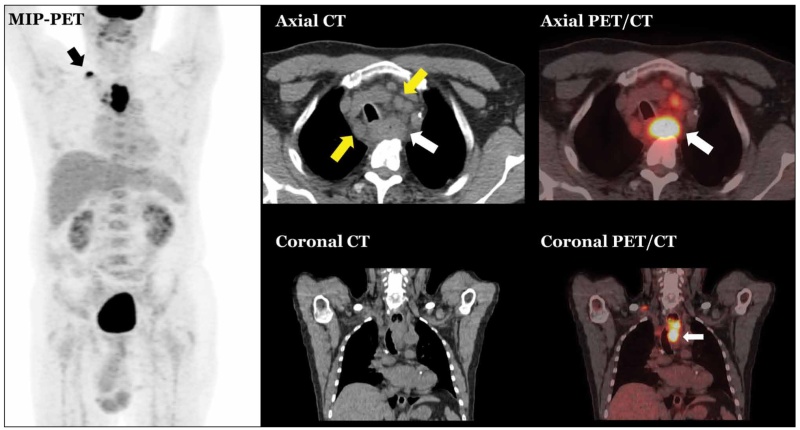 Figure 5. Advanced T-stage oesophageal cancer with nodal metastases. Primary tumor (white arrow) presenting as abnormal concentric wall thickening with adjacent peri-oesophageal fat stranding and displacement of the trachea to the right. There are FDG-avid nodes in the mediastinum (yellow arrow) and in the right supraclavicular fossa (black arrow).
Figure 5. Advanced T-stage oesophageal cancer with nodal metastases. Primary tumor (white arrow) presenting as abnormal concentric wall thickening with adjacent peri-oesophageal fat stranding and displacement of the trachea to the right. There are FDG-avid nodes in the mediastinum (yellow arrow) and in the right supraclavicular fossa (black arrow).
PET/CT it is not appropriate for the initial evaluation of primary pancreatic adenocarcinoma, because it cannot define by itself any encasement or infiltration of major vessels namely the superior mesenteric artery or the celiac axis. Of note that primary pancreatic adenocarcinomas show variable, sometimes low or moderate FDG uptake due to adjacent abundant fibrotic stroma, while inflammation-pancreatitis can be a source of false-positive findings. The role of PET/CT is reserved as a complementary tool in the assessment of probable metastatic disease and, in the restaging setting, to differentiate between metabolically active disease and post-treatment necrotic or fibrotic charges [38,39].
PET/CT is not suitable for the initial diagnosis and staging of hepatocellular cancer, because this malignancy shows heterogenous metabolic behavior with low FDG uptake in some cases due to low glucose transporter expression or due to increased phosphatase activity which metabolizes FDG.
Regarding colorectal cancer, PET/CT has limitations in T- and N-staging and strengths in the detection of distant metastases [35,40]. Primary tumor may appear as hypermetabolic abnormal mural thickening with concomitant luminal narrowing or as a polypoid intraluminal lesion. The modality may be useful in certain clinical scenarios such as: i) in selected candidate patients before the radical treatment of hepatic metastases. PET/CT may detect extrahepatic lesions altering treatment plan ii) in patients with equivocal CT/MRI findings which affect treatment decisions iii) in selected patients with high tumor marker levels in whom previous imaging is negative iv) in selected rectal cancer patients with high probability of distant metastatic disease v) in post-treatment rectal cancer, to evaluate residual pre-sacral tissue and differentiate between fibrosis or recurrent disease. In Gastrointestinal Stromal Tumors (GISTS), the method is useful in the evaluation of response to imatinib therapy not always fulfilling the typical criteria of tumor size reduction [41,42].
In gynecological cancers, PET/CT has certain, discrete roles. It is suitable for nodal and distal metastatic evaluation in cervical cancer and may modify treatment plan [43]. Thus, it is suggested by clinical guidelines in advanced-stage FIGO II-IV disease both for initial staging and for restaging/follow-up. In ovarian cancer, PET/CT has complementary role in staging of abdominopelvic nodes and distant metastases [44,45]. Of note that, ovarian cancer often metastasizes in the peritoneum in the form of peritoneal nodules, plaques and thickening or “haziness” of peritoneal fat. In endometrial cancer, PET/CT has high specificity in the detection of nodal disease and high diagnostic accuracy in detecting distant metastases; hence, it is applied and offers diagnostic benefit in high-risk patients [46].
PET/CT has no role in the initial diagnosis of breast cancer with very low sensitivity (≤ 50%) in primary breast cancer detection, low spatial resolution for the detection of small sub-centimeter lesions and considerable number of false positives in cases of inflammation, abscesses, fat necroses and fibroadenomas [47]. Despite all previous limitations, focal incidental PET-positive breast findings need further evaluation with mammography, because they bear a considerable probability of malignancy, around 30-40% based on retrospective studies. FDG uptake in the primary tumor depends on histopathological characteristics: lobular carcinomas show lower uptake than infiltrative ductal ones, while high-grade, highly proliferative and triple-negative tumors show intense uptake [48]. PET/CT has unacceptable, low sensitivity in the evaluation of axillary nodes (55-60%) and inability to detect micrometastatic burden, hence it cannot substitute for sentinel lymph node biopsy procedure, which is the standard of care in the evaluation of the axilla. The main strengths of PET/CT are: i) the potential to detect and depict hypermetabolic nodes beyond axillary levels I-II (typical nodal clearance is performed in these levels) and ii) the high sensitivity in distal metastatic evaluation even in sites not suspected by previous imaging. Thus, PET/CT is suggested in breast cancer stages ≥IIB-III and in triple-negative tumors [48-50]. It is not routinely suggested in early stage I-IIA tumors as it is unlikely to induce significant clinical impact. PET may detect hypermetabolic bone lesions with no corresponding anatomic abnormalities on the CT component of the study. Of note that breast skeletal metastases may, occasionally, appear sclerotic not showing increased metabolic activity.
In seminomas, PET/CT is suggested, by clinical guidelines, to evaluate any post-treatment residual tissue and differentiate between residual active neoplastic disease and necrotic scar tissue with high NPV>90% [51]. PET has very few clinical applications in renal-urinary cancers hampered by high normal FDG excretion into the urinary tract. It shows, however, high diagnostic accuracy in the detection of distal metastatic lesions from invasive bladder tumors [52]. In sarcomas, FDG activity in primary tumors depends on tumor grading [53]: osteosarcomas, Ewing sarcoma and high-grade chondrosarcoma show avid uptake, while high-grade liposarcomas show more intense uptake than myxoid and well-differentiated subtypes. Clinical guidelines include PET/CT in the staging of bone sarcomas and suggest this modality as complementary method in staging of soft-tissue sarcomas [54,55]. In research setting, PET/CT is applied to evaluate neoadjuvant chemotherapy and distinguish between responders and non-responders. The modality is also applied in sarcoma restaging to detect recurrent disease in areas with distorted anatomy by previous surgical treatment.
FDG dominates the clinical applications of PET in oncology, yet it is not the sole efficient radioactive tracer available. Other PET tracers following different molecular pathways rather than cellular metabolism or having high affinity for specific cellular receptors have been developed. These tracers have fulfilled unmet clinical needs and, nowadays, they have found their place into diagnostic and therapeutic algorithms.
Neuroendocrine tumors (NETs) over-express Somatostatin Receptors (SSTRs) on their cellular membranes. These receptors are effectively targeted with positron-emitting peptides (DOTA-octreotides e.g., 68Ga-DOTATATE), which show high selective affinity for SSTRs. DOTA-peptides are indicated in the staging and restaging of NETs, showing high diagnostic accuracy in mapping the whole disease burden, thus affecting therapeutic decisions [56]. DOTA-peptides have another inherent advantage in the evaluation of NETs: diagnostic verification of avid SSTR expression with PET provides the basis for further NET targeted treatment by use of the corresponding therapeutic DOTA-peptides (e.g., 177Lu-DOTATATE) emitting lethal beta radiation. The concept of applying the same molecular structure (octreotide) both for diagnosis and therapy is known as theranostics, a field where Nuclear Medicine meets Precision Medicine. The theranostic treatment of NETs by means of DOTA-peptides has been approved in clinical practice as it shows survival benefit verified by NETTER-1 randomized controlled trial [57].
Nowadays, various effective PET tracers, namely Prostate-Specific Membrane Antigen (PSMA)-ligands, are applied in the imaging of prostate cancer. PSMA is a specific trans-membranic glycoprotein with 100-1000fold over-expression in prostate cancer compared with normal prostatic tissue [58]. PSMA expression is particularly high in high-grade, metastatic and castration-resistant tumors. Specific PET tracers (i.e., 68Ga-PSMA-11, 18F-PSMA-1007) have been developed exhibiting high selective affinity for PSMA. PSMA-PET gives images of exceptional quality showing avid tracer uptake in cancerous lesions with high target to background ratios. It is the Nuclear Medicine method of choice for imaging of prostate cancer. PSMA-PET shows high sensitivity in the detection of small lesions such as sub-centimeter nodes and early bone lesions with no concomitant sclerosis. It is clinically useful in staging of high-risk patients showing higher diagnostic accuracy compared with conventional CT and bone scan imaging [59,60]. PSMA-PET is indicated in cases of biochemical recurrence with high detection rates even in low trigger PSA values <1 ng/ml [61]. In these patients, it may differentiate between loco-regional recurrence, which can be treated with radiotherapy, and metastatic disease, which is going to be subjected to systemic treatment. To evaluate local recurrence, MRI is still the modality of choice, while distal disease is more effectively assessed with PSMA-PET. In PSMA imaging, the theragnostic concept applies: metastatic lesions exhibiting avid tracer uptake may be, selectively, targeted with therapeutic 177Lu-PSMA. The latter emits beta radiation which destroys cancer cells and has already shown favorable results in clinical trials [62,63].
To sum up, PET/CT has evolved to be an established method in everyday oncological practice. It has been incorporated into clinical algorithms and guidelines altering therapeutic decisions in oncological patients. Besides the approved clinical indications, the quest for technological improvements, new applications and novel tracers continues, and the future of PET molecular imaging appears promising.
Conflict of interest disclosure
None to declare
Declaration of funding sources
None to declare
Author contributions
All authors contributed to the conception and design, analysis of the relevant literature, drafting of the article, critical revision for important intellectual content and final approval of the paper.
REFERENCES
1. Almuhaideb A, Papathanasiou N, Bomanji J. 18F-FDG PET/CT imaging in oncology. Ann Saudi Med. 2011; 31(1):3-13.
2. Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;4 (2):328-54.
3. Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47(5):885-95.
4. El-Galaly TC, Gormsen LC, Hutchings M. PET/CT for Staging; Past, Present, and Future. Semin Nucl Med. 2018;48(1):4-16.
5. Weiler-Sagie M, Kagna O, Dann EJ, Ben-Barak A, Israel O. Characterizing bone marrow involvement in Hodgkin’s lymphoma by FDG-PET/CT. Eur J Nucl Med Mol Imaging. 2014; 41(6):1133-40.
6. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-68.
7. Eichenauer DA, Engert A, Andre M, Federico M, Illidge T, Hutchings M, et al. Hodgkin’s lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014; 25 Suppl 3:iii70-5.
8. Fuertes S, Setoain X, Lopez-Guillermo A, Montserrat E, Fuster D, Paredes P, et al. [The value of positron emission tomography/computed tomography (PET/CT) in the staging of diffuse large B-cell lymphoma]. Med Clin (Barc). 2007;129(18):688-93.
9. Gallamini A, Zwarthoed C. Interim FDG-PET Imaging in Lymphoma. Semin Nucl Med. 2018;48(1):17-27.
10. Andre MPE, Girinsky T, Federico M, Reman O, Fortpied C, Gotti M, et al. Early Positron Emission Tomography Response-Adapted Treatment in Stage I and II Hodgkin Lymphoma: Final Results of the Randomized EORTC/LYSA/FIL H10 Trial. J Clin Oncol. 2017;35 (16):1786-94.
11. Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372 (17):1598-607.
12. Johnson P, Federico M, Kirkwood A, Fossa A, Berkahn L, Carella A, et al. Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin’s Lymphoma. N Engl J Med. 2016;374(25):2419-29.
13. Barrington SF, Kluge R. FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):97-110.
14. Kobe C, Dietlein M, Hellwig D. PET/CT for Lymphoma Post-therapy Response Assessment in Hodgkin Lymphoma and Diffuse Large B-cell Lymphoma. Semin Nucl Med.2018;48(1):28-36.
15. Karls S, Shah H, Jacene H. PET/CT for Lymphoma Post-therapy Response Assessment in Other Lymphomas, Response Assessment for Autologous Stem Cell Transplant, and Lymphoma Follow-up. Semin Nucl Med. 2018;48(1):37-49.
16. Niyonkuru A, Bakari KH, Lan X. (18)F-Fluoro-2-Deoxy-d-Glucose PET/Computed Tomography Evaluation of Lung Cancer in Populations with High Prevalence of Tuberculosis and Other Granulomatous Disease. PET Clin. 2018;13(1):19-31.
17. Truong MT, Viswanathan C, Carter BW, Mawlawi O, Marom EM. PET/CT in the thorax: pitfalls. Radiol Clin North Am. 2014;52(1):17-25.
18. Liu Y. Lung Neoplasms with Low F18-Fluorodeoxyglucose Avidity. PET Clin. 2018;13(1):11-8.
19. Akhurst T. Staging of Non-Small-Cell Lung Cancer. PET Clin. 2018;13(1):1-10.
20. Gao SJ, Kim AW, Puchalski JT, Bramley K, Detterbeck FC, Boffa DJ, et al. Indications for invasive mediastinal staging in patients with early non-small cell lung cancer staged with PET-CT. Lung Cancer. 2017;109:36-41.
21. Wang J, Welch K, Wang L, Kong FM. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer. 2012;13(2):81-9.
22. Goel R, Moore W, Sumer B, Khan S, Sher D, Subramaniam RM. Clinical Practice in PET/CT for the Management of Head and Neck Squamous Cell Cancer. AJR Am J Roentgenol. 2017;209(2):289-303.
23. Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer. 2004; 101(11):2641-9.
24. Sanli Y, Zukotynski K, Mittra E, Chen DL, Nadel H, Niederkohr RD, et al. Update 2018: 18F-FDG PET/CT and PET/MRI in Head and Neck Cancer. Clin Nucl Med. 2018;43 (12):e439-52.
25. Strohl MP, Ha PK, Flavell RR, Yom SS. PET/CT in Surgical Planning for Head and Neck Cancer. Semin Nucl Med. 2021;51(1):50-8.
26. Wong WL. PET-CT for Staging and Detection of Recurrence of Head and Neck Cancer. Semin Nucl Med. 2021;51(1):13-25.
27. Haerle SK, Schmid DT, Ahmad N, Hany TF, Stoeckli SJ. The value of (18)F-FDG PET/CT for the detection of distant metastases in high-risk patients with head and neck squamous cell carcinoma. Oral Oncol. 2011;47(7):653-9.
28. Loo SW, Geropantas K, Beadsmoore C, Montgomery PQ, Martin WM, Roques TW. Neck dissection can be avoided after sequential chemoradiotherapy and negative post-treatment positron emission tomography-computed tomography in N2 head and neck squamous cell carcinoma. Clin Oncol (R Coll Radiol). 2011;23(8):512-7.
29. Rogers JW, Greven KM, McGuirt WF, Keyes JW, Jr., Williams DW, 3rd, Watson NE, et al. Can post-RT neck dissection be omitted for patients with head-and-neck cancer who have a negative PET scan after definitive radiation therapy? Int J Radiat Oncol Biol Phys. 2004;58 (3):694-7.
30. Fugazzola L, Elisei R, Fuhrer D, Jarzab B, Leboulleux S, Newbold K, et al. 2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur Thyroid J. 2019; 8(5):227-45.
31. Perng P, Marcus C, Subramaniam RM. (18)F-FDG PET/CT and Melanoma: Staging, Immune Modulation and Mutation-Targeted Therapy Assessment, and Prognosis. AJR Am J Roentgenol. 2015;205(2):259-70.
32. Hafner J, Schmid MH, Kempf W, Burg G, Kunzi W, Meuli-Simmen C, et al. Baseline staging in cutaneous malignant melanoma. Br J Dermatol. 2004;150(4):677-86.
33. Hicks RJ, Iravani A, Sandhu S. (18)F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for Assessing Tumor Response to Immunotherapy in Solid Tumors: Melanoma and Beyond. PET Clin. 2020;15(1):11-22.
34. Mena E, Sanli Y, Marcus C, Subramaniam RM. Precision Medicine and PET/Computed Tomography in Melanoma. PET Clin. 2017;12(4):449-58.
35. Akin EA, Qazi ZN, Osman M, Zeman RK. Clinical impact of FDG PET/CT in alimentary tract malignancies: an updated review. Abdom Radiol (NY). 2020;45(4):1018-35.
36. Lerut T, Flamen P, Ectors N, Van Cutsem E, Peeters M, Hiele M, et al. Histopathologic validation of lymph node staging with FDG-PET scan in cancer of the esophagus and gastroesophageal junction: A prospective study based on primary surgery with extensive lymphadenectomy. Ann Surg. 2000;232(6):743-52.
37. Kim TJ, Kim HY, Lee KW, Kim MS. Multimodality assessment of esophageal cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2009;29(2):403-21.
38. Pinho DF, Subramaniam RM. PET-Computed Tomography and Precision Medicine in Pancreatic Adenocarcinoma and Pancreatic Neuroendocrine Tumors. PET Clin. 2017;12 (4):407-21.
39. Zins M, Matos C, Cassinotto C. Pancreatic Adenocarcinoma Staging in the Era of Preoperative Chemotherapy and Radiation Therapy. Radiology. 2018;287(2):374-90.
40. Agarwal A, Marcus C, Xiao J, Nene P, Kachnic LA, Subramaniam RM. FDG PET/CT in the management of colorectal and anal cancers. AJR Am J Roentgenol. 2014; 203(5):1109-119.
41. Hess S, Bjerring OS, Pfeiffer P, Hoilund-Carlsen PF. Personalized Clinical Decision Making in Gastrointestinal Malignancies: The Role of PET. PET Clin. 2016;11(3):273-83.
42. Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv68-78.
43. Gandy N, Arshad MA, Park WE, Rockall AG, Barwick TD. FDG-PET Imaging in Cervical Cancer. Semin Nucl Med. 2019;49(6):461-70.
44. Kemppainen J, Hynninen J, Virtanen J, Seppanen M. PET/CT for Evaluation of Ovarian Cancer. Semin Nucl Med. 2019;49(6):484-92.
45. Khiewvan B, Torigian DA, Emamzadehfard S, Paydary K, Salavati A, Houshmand S, et al. An update on the role of PET/CT and PET/MRI in ovarian cancer. Eur J Nucl Med Mol Imaging. 2017;44(6):1079-91.
46. Kilcoyne A, Chow DZ, Lee SI. FDG-PET for Assessment of Endometrial and Vulvar Cancer. Semin Nucl Med. 2019;49 (6):471-83.
47. Adejolu M, Huo L, Rohren E, Santiago L, Yang WT. False-positive lesions mimicking breast cancer on FDG PET and PET/CT. AJR Am J Roentgenol. 2012;198(3):W304-14.
48. Groheux D, Espie M, Giacchetti S, Hindie E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013; 266(2):388-405.
49. Groheux D, Cochet A, Humbert O, Alberini JL, Hindie E, Mankoff D. (1)(8)F-FDG PET/CT for Staging and Restaging of Breast Cancer. J Nucl Med. 2016;57 Suppl 1:17S-26S.
50. Ulaner GA. PET/CT for Patients With Breast Cancer: Where Is the Clinical Impact? AJR Am J Roentgenol. 2019;213(2):254-65.
51. Oldenburg J, Fossa SD, Nuver J, Heidenreich A, Schmoll HJ, Bokemeyer C, et al. Testicular seminoma and non-seminoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi125-32.
52. Bagheri MH, Ahlman MA, Lindenberg L, Turkbey B, Lin J, Cahid Civelek A, et al. Advances in medical imaging for the diagnosis and management of common genitourinary cancers. Urol Oncol. 2017;35(7):473-91.
53. Costelloe CM, Chuang HH, Madewell JE. FDG PET/CT of primary bone tumors. AJR Am J Roentgenol. 2014;202(6):W521-31.
54. Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv51-67.
55. Casali PG, Bielack S, Abecassis N, Aro HT, Bauer S, Biagini R, et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv79-95.
56. Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37(10):2004-10.
57. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376(2):125-35.
58. Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiographics. 2018;38(1):200-17.
59. Hofman MS. ProPSMA: A Callout to the Nuclear Medicine Community to Change Practices with Prospective, High-Quality Data. J Nucl Med. 2020;61(5):676-7.
60. Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208-16.
61. Barbosa FG, Queiroz MA, Nunes RF, Viana PCC, Marin JFG, Cerri GG, et al. Revisiting Prostate Cancer Recurrence with PSMA PET: Atlas of Typical and Atypical Patterns of Spread. Radiographics. 2019;39(1):186-212.
62. Giraudet AL, Kryza D, Hofman M, Moreau A, Fizazi K, Flechon A, et al. PSMA targeting in metastatic castration-resistant prostate cancer: where are we and where are we going? Ther Adv Med Oncol. 2021;13:17588359211053898.
63. Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.