ACHAIKI IATRIKI | 2020; 39(2): 63–65
Editorial
Aikaterini Patsatsi
Autoimmune Bullous Diseases Unit, 2nd Dermatology Department, Aristotle University Faculty of Medicine, Papageorgiou General Hospital, Thessaloniki, Greece
Received: 25 March 2020; Accepted: 4 May 2020
Corresponding author: Aikaterini Patsatsi, MD, MSc, PhD, Associate Professor of Dermatology & Venereology Autoimmune Bullous Diseases Unit, 2nd Dermatology Department, Aristotle University Faculty of Medicine, Papageorgiou General Hospital, Thessaloniki, Greece, E-mail: apatsats@auth.gr
Key words: Bullous pemphigoid, gliptins, dipeptylpeptidase– 4 inhibitors, DPP-4i
Bullous pemphigoid (BP) is an autoantibody – medi- ated organ specific autoimmune disorder and is con- sidered as the most common autoimmune bullous disease in the elderly population globally [1, 2]. BP typically presents with pruritic erythematous or urticarial plaques and tense blisters on the trunk and extremities (Figure 1a-b).
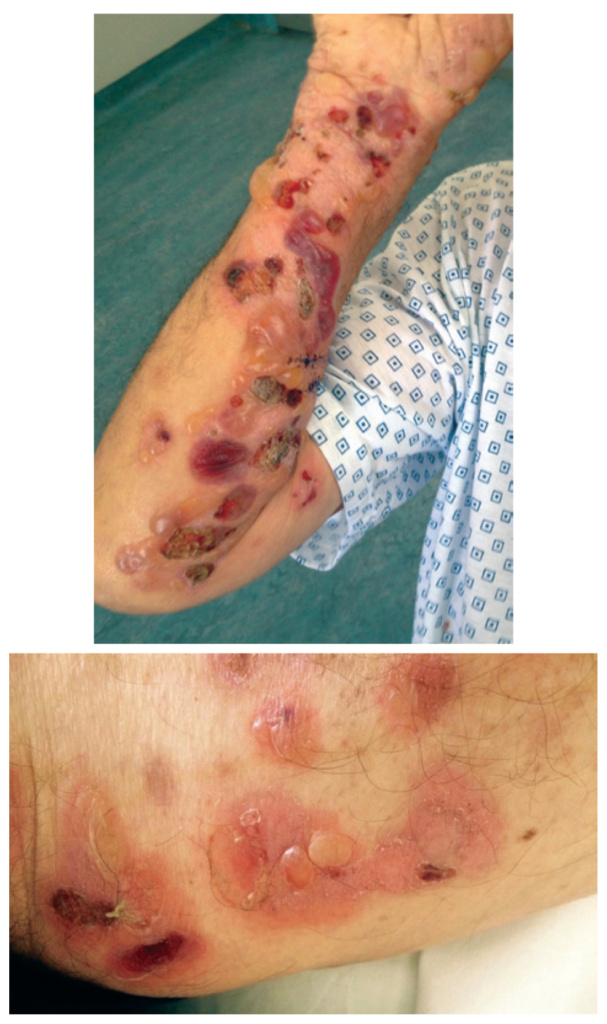
Figure 1(a-b). Erythematous plaques and tense blisters on the extremities
There are still gaps in the precise description of blister formation in BP. It is suggested that triggering factors induce loss of immunotolerance against adhe- sion molecules of the skin, followed by a cascade of events leading to the formation of circulating and tissue bound autoantibodies against target antigens of the basement membrane zone. The target antigens in BP are mainly BP180 (BP80NC16a domain) and BP230, both structural proteins of the hemidesmosomes, located at the dermal – epidermal junction [1]. According to the current knowledge of BP pathogenetic chain, once autoantibodies are bound to the basement membrane zone, there is activation of complement, degranulation of mast cells and release of leukotrienes, TNF- a and other cytokines [1, 2]. The inflammatory cascade continues with the activation of neutrophils and eosinophils, fol- lowed by the production of proteolytic enzymes which induce degradation of the dermal epidermal junction and blister formation [1, 2].
There is a growing incidence of bullous pemphigoid worldwide and one of the possible explanations, apart from the increasing life expectancy of the population, is the increasing use of culprit medications [2, 3]. It is possible that certain drugs trigger the breakage of im- munotolerance against especially BP180, induce the production of autoantibodies and initiate the blister formation process. Up to now, there are more than 60 drugs associated with BP [3]. Among them, dipeptyl- peptidase – 4 inhibitors (DPP-4i or gliptins) carry the highest risk. There is a global concern, as these category of antidiabetic drugs are quite effective and thus widely prescribed [3, 4].
The first case series of a possible association of gliptins with induction of BP were published by Greek centers, one from Patra and one from Ioannina, Greece [5, 6]. In 2016, Garcia et al identified 170 cases of BP in patients taking a DPP-4i in the European Pharmacovigi- lance database, suggesting that the intake of DPP-4i was more frequently associated with the development of BP when compared with that of other drugs [7]. A disproportionally high number of cases were linked to vildagliptin use [7]. Another report during the same year, from the French Pharmacovigilance database recorded all spontaneous reports of DPP-4i-related BP cases between April 2008 and August 2014 and provided evidence supporting an increased risk of development of BP associated with DPP-4i exposure, especially with vildagliptin exposure [8]. The increased risk of develop- ing BP among diabetic patients under treatment with DPP-4 was clearly shown in one of the largest retro- spective, nationwide, population-based, case-control studies from Korea. The authors suggest that the use of DPP-4 inhibitors is associated with the development of BP in patients with diabetes and particularly the use of vildagliptin in male patients [9].
The pathogenesis of DPP-4i-associated BP remains largely unclear. Dipeptylpeptidase IV (also known as CD26) is an aminopeptidase expressed in various types of cells. It acts also as a cell surface antigen present on T lymphocytes (CD26) and thus, it may be reasonable to consider that the inhibition of CD26 expression on T- cells may provoke an immune response [10]. Another element under consideration is that DPP-4 is a cell-sur- face plasminogen receptor that converts plasminogen to plasmin. Plasmin is a major serine protease which may cleave BP180 into its 120 and 97 kD ectodomains and therefore, suppression of DPP-4 may be associated with the development of novel epitopes for DPP-4i-BP autoantibodies [3, 11]. Additionally, inhibition of DPP-4 induces the infiltration of eosinophils into the skin, a key – cell population in BP pathogenesis [12]. It is also interesting that selective BP180 immunotolerance break- age by DPP-4i exposure is reported in HLA-DQB1_03:01 Japanese carriers [13].
Ιn the current literature there is a controversy regard- ing clinical and immunological features of the so-called “gliptin induced pemphigoid”. In studies from Japan there is evidence that this is a distinct, recently described form of BP with a less inflammatory phenotype, fewer infiltrating eosinophils in lesional skin, a different patho- genic epitope – the extracellular non-NC16A region of BP180 outside the NC16A domain – and predilection for certain HLAs [14, 15].
On the other hand, according to European studies, gliptins just trigger the development of bullous pem- phigoid and there is no need to describe a separate clinical form of the disease. In a multicenter retrospec- tive case – control study from France and Switzerland, it was shown that there were no apparent differences in clinical presentation and initial management be- tween patients with diabetes and BP who had been treated with DPP-4i and patients with diabetes and BP who had not been treated with DPP-4i [16]. In our center in Thessaloniki, we performed a retrospective study in a sample of 143 BP patients and we found no differences in the clinical and immunological features, apart from a higher number of relapses in patients with classic BP [17].
One of the critical questions regarding the induction of a certain eruption by any drug is the time of exposure. According to Tan et al (2017) in order for a drug to be a trigger for BP it should be (i) started within 1 year preceding the diagnosis of BP, (ii) taken for more than 2 weeks and (iii) not stopped for more than 1 month prior to diagnosis [18]. Relevant literature shows that there is a long latency period between the initiation of gliptin treatment and development of BP in the majority of cases, leading to the assumption that gliptins aggravate and not really induce BP [3].
Quite recently, an analysis based on pharmacovigi- lance databases provided clear evidence of a BP-DPP-4i association [19]. Nevertheless, elderly patients take various medications and have other concomitant dis- eases. A rather reasonable question to be answered is whether DPP-4i alone are sufficient to induce BP or other factors are also required to induce breakdown of immunotolerance to BP180.
In conclusion, there is sufficient evidence nowadays regarding the association of DPP-4 inhibitors and BP in elderly diabetic patients. Although there are still gaps in the interpretation of gliptin associated BP, in the detailed description of phenotype, immunological profile and overall course, it is necessary to replace this pharma- cological class with other antidiabetic medication in diabetic patients with bullous pemphigoid.
Conflict of interest disclosure
None to declare
Declaration of funding sources
None to declare
References
1. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320-32.
2. Genovese G, Di Zenzo G, Cozzani E, Berti E, Cugno M, Mar- zano AV. New Insights Into the Pathogenesis of Bullous Pemphigoid: 2019 Update. Front Immunol. 2019;10:1506.
3. Tasanen K, Varpuluoma O, Nishie W. Dipeptidyl Peptidase-4 Inhibitor-Associated Bullous Pemphigoid. Front Immunol. 2019;10:1238
4. Lee SG, Lee HJ, Yoon MS, Kim DH. Association of Dipeptidyl Peptidase 4 Inhibitor Use With Risk of Bullous Pemphigoid in Patients With Diabetes. JAMA Dermatol. 2019;155(2):172-7.
5. Pasmatzi E, Monastirli A, Habeos J, Georgiou S, Tsambaos
D. Dipeptidyl peptidase-4 inhibitors cause bullous pem- phigoid in diabetic patients: report of two cases. Diabetes Care. 2011;34(8):e133.
6. Skandalis K, Spirova M, Gaitanis G, Tsartsarakis A, Bassukas ID. Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. 2012;26(2):249-53.
7. García M, Aranburu MA, Palacios-Zabalza I, Lertxundi U, Aguirre C. Dipeptidyl peptidase-IV inhibitors induced bullous pemphigoid: a case report and analysis of cases reported in the European pharmacovigilance database. J Clin Pharm Ther. 2016;41(3):368-70.
8. Béné J, Moulis G, Bennani I, Auffret M, Coupe P, Babai S, et al. Bullous pemphigoid and dipeptidyl peptidase IV inhibi- tors: a case-noncase study in the French Pharmacovigilance Database. Br J Dermatol. 2016;175(2):296-301.
9. Lee SG, Lee HJ, Yoon MS, Kim DH. Association of Dipeptidyl Peptidase 4 Inhibitor Use With Risk of Bullous Pemphigoid in Patients With Diabetes. JAMA Dermatol. 20191;155(2):172-7.
10. Ohnuma K, Dang NH, Morimoto C. Revisiting an old ac- quaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008;29(6):295-301.
11. Hofmann SC, Voith U, Schönau V, Sorokin L, Bruckner- Tuderman L, Franzke C. Plasmin plays a role in the in vitro generation of the linear IgA dermatosis antigen LADB97. J Invest Dermatol. 2009;129(7):1730-9.
12. Forssmann U, Stoetzer C, Stephan M, Kruschinski C, Skripu- letz T, Schade J. Inhibition of CD26/dipeptidyl peptidase IV enhances CCL11/eotaxinmediated recruitment of eosino- phils in vivo. J Immunol. 2008;181(2):1120-7.
13. Ujiie H, Muramatsu K, Mushiroda T, Ozeki T, Miyoshi H, Iwata
H. HLA-DQB1_ 03: 01 as a biomarker for genetic susceptibil- ity to bullous pemphigoid induced by DPP-4 inhibitors. J Invest Dermatol. 2018;138(5):1201-4.
14. Izumi K, Nishie W, Mai Y, Wada M, Natsuga K, Ujiie H, et al. Autoantibody Profile Differentiates between Inflamma- tory and Noninflammatory Bullous Pemphigoid. J Invest Dermatol. 2016;136(11):2201-10.
15. Nishie W. Dipeptidyl peptidase IV inhibitor-associated bullous pemphigoid: a recently recognized autoimmune blistering disease with unique clinical, immunological and genetic characteristics. Immunol Med. 2019;42(1):22-8.
16. Benzaquen M, Borradori L, Berbis P, Cazzaniga S, Valero R, Richard MA, et al. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: Retrospective multicenter case-control study from France and Switzerland. J Am Acad Dermatol. 2018;78(6):1090-6.
17. Patsatsi A, Kyriakou A, Meltzanidou P, Trigoni A, Lamprou F, Kokolios M, et al. Βullous pemphigoid in patients with DPP-4 inhibitors at the onset of disease: does this dif- fer from common bullous pemphigoid? Eur J Dermatol. 2018;28(5):711-3.
18. Tan CW, Pang Y, Sim B, Thirumoorthy T, Pang SM, Lee HY. The association between drugs and bullous pemphigoid. Br J Dermatol. 2017;176(2):549-51.
19. Molina-Guarneros JA, Sainz-Gil M, Sanz-Fadrique R, García P, Rodríguez-Jiménez P, Navarro-García E, et al. Bullous pem- phigoid associated with the use of dipeptidil peptidase-4 inhibitors: analysis from studies based on pharmacovigi- lance databases. Int J Clin Pharm. 2020;42(2):713-20.