ACHAIKI IATRIKI | 2021; 40(1):28–33
Original research article
Maria Rodi, Tassos Georgakopoulos, Fotini Kalogianni, Athena Alexandropoulou, Anne-Lise de Lastic, Ioannis Panagoulias, Athanasia Mouzaki
Laboratory of Molecular Diagnosis of Infectious Agents, Medical School, University of Patras, Patras, Greece
Received: 30 Nov 2020; Accepted: 18 Jan 2020
Corresponding author: Athanasia Mouzaki, Laboratory of Molecular Diagnosis of Infectious Agents, Medical School University of Patras, Patras, Greece Tel.: +30 2610 969123, E-mail: mouzaki@upatras.gr, ORCID: 0000-0001-5548-7002
Key words: COVID-19, RNA extraction, quantitative real time PCR
Abstract
SARS-CoV-2, the virus responsible for the ongoing COVID-19 outbreak, is a positive single-stranded RNA virus similar to existing SARS-CoV viruses, although evolutionarily distant. To date, diagnosis for COVID-19 has been based on the detection of SARS-CoV-2 in nasopharyngeal or oropharyngeal specimens using quantitative real-time PCR (qRT-PCR). The mortality rates and rapid spread of SARS-CoV-2 have led to a high demand for commercially available kits and reagents for the extraction of viral RNA and subsequent qRT-PCR analysis. From the experience of many laboratories, including our own, there is often a shortage of kits, particularly those for viral RNA extraction. We report here a protocol we developed for RNA extraction for the detection of SARS-CoV-2 by qRT-PCR. Our method uses reagents commonly found in molecular biology research laboratories that are readily available and inexpensive. Comparison of our in-house method with an automated total RNA extraction method using a widely available commercial kit showed that we consistently obtained the same or better results with our in-house RNA extraction protocol.
Introduction
The novel coronavirus SARS-CoV-2, which emerged in Wuhan, China, since December 2019, has spread rapidly worldwide, infecting over 94.5 million people, of whom 2,022,279 have died [1].
SARS-CoV-2 is a positive single-stranded RNA virus that is similar to previous SARS-CoV viruses, although it is evolutionarily distant. The SARS-CoV-2 ssRNA encodes proteins with distinct roles, including the spike glycoprotein, which is responsible for cell adhesion and entry via the host cell receptor ACE2, and Nsp1, which acts as an antagonist of the IFN response and induces chemokine secretion [2-6].
In patients with COVID-19, lymphopenia, mainly due to activation-induced T-cell death, accompanied by reduced numbers of regulatory T-cells and significant upregulation of the cytokines IL-1β, IL-6, TNF-α, IL-10 and the chemokines MCP1, IP-10, MIP1A, MIP1B, is associated with poor outcome [7,8].
To date, diagnosis has been based mainly on the detection of SARS-CoV-2 in nasopharyngeal or oropharyngeal specimens by qRT-PCR [9]. The mortality rates and rapid spread of SARS-CoV-2 led to a high demand for commercially available kits and reagents for viral RNA extraction and qRT-PCR analysis. From the experience of many laboratories, including our own, there is often a shortage of kits and reagents, particularly those for viral RNA extraction. We report here a protocol that we have developed in our laboratory for SARS-CoV-2 RNA extraction to be used for SARS-CoV-2 detection by qRT-PCR. Our method uses reagents commonly found in molecular biology research laboratories that are readily available and inexpensive.
Materials and methods
Ethics
The Laboratory of Molecular Diagnosis of Infectious Agents, Medical School, University of Patras, Patras, Greece, is one of the COVID-19 reference laboratories in Greece that performs SARS-CoV-2 detection by qRT-PCR in nasopharyngeal and oropharyngeal samples collected by hospital staff or health care workers.
The Laboratory performs these tests for the 6th Health District of Greece (Southwest Greece and Ionian Sea islands). The Laboratory was established by Resolution no. 217/9186/12.3.2020 of the Extraordinary Session of the Senate of the University of Patras 164/12.3.2020, which was published in the Government Gazette on 21/03/2020, no. 955.
The methodology described in this study and the data analyzed are part of a service provided by the Laboratory to the Hellenic National Public Health Organization (EODY). EODY and the Greek government encourage the official COVID-19 laboratories to develop their own protocols for RNA extraction and SARS-CoV-2 detection by qRT-PCR, to increase the diagnostic adequacy of the country in case of shortages of commercially available kits and reagents for viral RNA extraction and qRT-PCR analysis.
Case demographics and test results are sent electronically to EODY in encrypted files that can be opened with a code known only to the laboratory director and designated EODY staff. This procedure has been approved by the Hellenic Data Protection Authority. For the purpose of the current study, informed consent was not required.
Samples
Nasopharyngeal and oropharyngeal samples are collected in tubes containing Dulbecco’s Modified Eagle’s Medium (DMEM) (Copan swabs #330C USA; Deltalab Swab Virus #304297 and #304291 Spain; MicroBiotech swabs 250/VIR/AL, Italy) and stored at 4oC until they reach our laboratory. Samples are either processed immediately or stored at -80oC until processing. Sample handling and RNA extraction takes place in a biosafety level III laboratory in a type II hood in a clean room. qRT-PCR analysis takes place in a separate laboratory. Leftover biological and RNA samples are stored at -80oC. For this work, 6 frozen biological samples were used, including 4 positive and 2 negative for SARS-CoV-2, as previously determined using commercial kits, as described below.
In-house method for total RNA extraction
An amount of 300 μl per biological sample was transferred to a 1.5 ml Eppendorf-type centrifuge tube containing 700 μl Trizol (T9424 Sigma, Germany), mixed x5 by inversion, and left to stand for 5 min at RT. Next, 200 μl chloroform was added, mixed x5 by inversion, and allowed to stand for 3 min at RT. The tubes were centrifuged at 12,000g at 4oC for 15 min. From the supernatants, 500-600 μl/sample were transferred to new tubes to which 600 μl of ice-cold isopropanol was added. The tubes were mixed x5 by inversion and allowed to stand at -80oC for 10 min. The tubes were then centrifuged at 12,000g at 4oC for 10 min. After removing the supernatant, RNA pellets were diluted in 75% ice-cold ethanol, and the tubes were centrifuged at 12,000g at 4oC for 5 min. The ethanol was removed and the RNA pellets were dried for 10 min at RT. Next, 12 μl of nano-pure water was added to each tube, and the tubes were incubated at 37oC for 10-15 min. The RNA concentration and purity in the aqueous solution was determined using a micro-volume UV-Vis Spectrophotometer Q5000 (Quawell Technology, Inc. USA). The method in protocol format is shown in Table 1.
Automated total RNA extraction
Automated total RNA extraction was performed with the Q-QiaSymphony Nucleic Acid extractor (Qiagen, Germany) using the QIA.937036 QIAsymphony DSP Virus/Pathogen Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions [10, 11]. The final volume of each RNA sample was 50 μl. For the purpose of this study RNA concentration and purity were determined as described above.
cDNA preparation and quantitative RT-PCR
The preparation of cDNAs from both in-house and automated extracted RNAs and subsequent qRT-PCR was performed using the one-step reverse transcription and RT-PCR detection kit VS-NCO296 VIASURE SARS-CoV-2 (CerTest Biotec, S.L., Spain) [12]. For the reaction, 5 μl of each RNA sample was added to 15 μl of the rehydrated reaction mix of the kit containing all the necessary factors for reverse transcription and PCR. Reverse transcription and qRT-PCR were performed on a Rotor-Gene Q MDx 5plex Platform RT-PCR cycler (Qiagen, Germany). The program for the reactions was: 15 min at 45ºC for reverse transcription, 2 min at 95ºC for initial denaturation of cDNAs and then for 45 cycles the following two steps, 10 sec at 95oC for denaturation and 50 sec at 60oC for annealing/extension.
Results and discussion
We compared the qRT-PCR results of 6 total RNAs extracted from biological samples using either QIAsymphony or our own method. Total RNAs extracted with QIAsymphony were in a total volume of 50 μl/sample. For the in-house method, total RNA was first diluted in a small volume (12 μl/sample), and serial dilutions were performed. The concentration of all RNA samples was determined spectrophotometrically (Table 2).
As shown in Table 2, the initial concentrations (A) of all RNAs prepared by the in-house method were higher than those prepared by QIAsymphony. The initial concentrations (A) of RNAs prepared by the in-house method were between 10-fold (P1) and 13-fold (P3) higher than those prepared by QIAsymphony. The final dilution concentrations (D) of the RNAs prepared in-house were closer to the concentrations of the RNAs prepared with QIAsymphony. Using the in-house method, extraction of total RNA takes approximately 1 h and 15 min for 6 biological samples.
For each qRT-PCR reaction, 5μl per RNA sample (diluted or undiluted) was used. Detection of SARS-CoV-2 using Viasure Real Time PCR Detection Kit is based on amplification of a conserved region of ORF1ab and N genes of SARS-CoV-2 with specific primers and fluorescent-labeled probes. In the Rotor-Gene Q MDx RT-PCR cycler, the N gene is amplified and detected in the ROX channel, the ORF1ab gene in the FAM channel, and the internal control (IC) in the HEX channel.
As shown in Table 3, sample P3 did not yield results for the virus N and ORF1ab genes or the internal control at the initial concentration (A); this sample yielded positive qRT-PCR results at lower concentrations. In reverse transcription reactions it is common to have a decrease of efficiency due to an excess of the starting template. High concentration templates probably contain greater amounts of reverse transcription and/or PCR inhibitors that limit subsequent steps.
Four RNA samples yielded positive results and 2 negative, at all concentrations used. Regardless of the RNA preparation method used or its concentration, the cycle thresholds (ct) were nearly identical for all targets tested (N gene, ORF ab gene and IC) (Table 3). In Figure 1, we show characteristic results of qRT-PCR of 2 positive samples (P1, P3) and 1 negative sample (N).
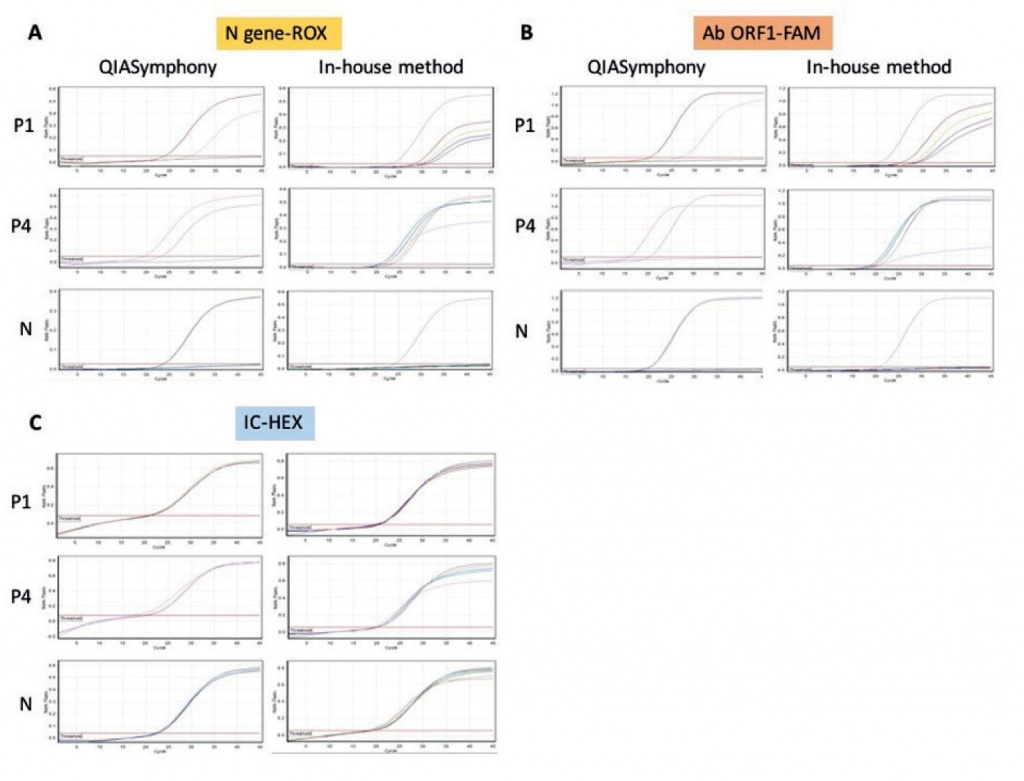
Figure 1. Representative qRT-PCR results using RNAs from 2 positive samples (P1, P4) and 1 negative sample (N) prepared either with an automated method or with our in-house method. A: N target gene of SARS-CoV-2, amplified and detected in the ROX channel; B: ORF1ab gene of SARS-CoV-2, amplified and detected in the FAM channel; C: internal control, amplified and detected in the HEX channel (IC). The actual ct values are shown in Table 3.
We repeated the in-house RNA extraction procedure for an additional 200 randomly selected positive and negative samples that we had in stock. For the additional samples, we decided to use a range of RNA concentrations between 1.2-264.2 ng/ml of sample (median concentration, 116.59 ng/ml) and we obtained the same results obtained with the QIAsymphony method. In addition, we were able to extract RNA from biological samples that could not be used for the QIAsymphony method due to too high viscosity or too low volume.
Conclusions
In conclusion, our in-house method for RNA extraction is standardized to perform as well as or better than a widely used automated method for RNA extraction. Our method uses reagents commonly found in molecular biology research laboratories that are readily available and cheap to buy, and it yields higher amounts of total RNA, which can be very helpful when the quality of biological samples is poor and/or their quantity is very low.
So far, massive screening of the population for SARS-CoV-2 by qRT-PCR combined with a policy of social distancing is the only protection against the infection. As vaccination against SARS-CoV-2 [13, 14] begins, molecular screening will continue to determine whether herd immunity develops and whether vaccinated individuals are immune to new SARS-CoV-2 infections.
Conflict of interest disclosure: None to declare.
Declaration of funding sources: This work was funded by the University of Patras Special Account for Research Funds Program Coronavirus COVID-19 #81198.
Author contributions: AM conceived and coordinated the study, MR, FK, AA, ALdL performed the experiments, TG advised on methodology, MR and TG analyzed the data and prepared the tables and figure, MR, TG, IP and AM wrote the manuscript. AM revised the manuscript. All authors approved the revised manuscript.
References
- Johns Hopkins Coronavirus Resource Center. “COVID-19 Map.” Accessed January 17, 2021. Available from: https://coronavirus.jhu.edu/map.html.
- Chan JF, Kok KH, Zhu Z, Chu H, To KW, Yuan S, et al. Genomic characterization of the 2019 novel human pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221-236.
- Andersen KG, Rambaut A, Lipkin WI, Holmes, EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450-452.
- Anastasopoulou S, Mouzaki A. The biology of SARS-CoV-2 and the ensuing COVID-19. Achaiki Iatriki. 2020;39(1):29-35.
- Stancioiu F, Papadakis G, Kteniadakis S, Nikovaevich Izotov B, Coleman M, Spandidos D, et al. A dissection of SARS‑CoV2 with clinical implications. Int J Mol Med. 2020;46(2):489-508.
- Docea A, Tsatsakis A, Albulescu D, Cristea O, Zlatian O, Vinceti M, et al. A new threat from an old enemy: Re‑emergence of coronavirus. Int J Mol Med. 2020;45(6):1631-1643.
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762-768.
- Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26(10):1636-1643.
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045.
- QIAsymphony SP Protocol Sheet- Complex200_OBL_V4_DSP protocol 12/2017 www.giagen.com
- QIAsymphony DSP Virus/Pathogen Mini Kit handbook 06/2015 www.giagen.com
- VS-NCO296 VIASURE SARS-CoV-2 RT-PCR Detection Kit handbook www.certest.es
- Koirala A, Joo YJ, Khatami A, Chiu C, Britton PN. Vaccines for COVID-19: The current state of play. Paediatr Respir Rev. 2020;35:43-49.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N Engl J Med. 2020;383(27):2603-2615.