ACHAIKI IATRIKI | 2022; 41(1): 7–12
Editorial
Andreas G Tzakis
Cleveland Clinic, 9500 Euclid Avenue, Cleveland, Ohio, United States
Received: 04 Oct 2021; Accepted: 03 Dec 2021
Corresponding author: Andreas G Tzakis, MD, PhD, Dhc (mult)* *Emeritus Director Transplantation, Cleveland Clinic Enterprise Member of the Academy of Athens
Key words: Transplant, Uterus Transplantation
Introduction
Transplantation of the uterus (UTx) concluded the list of the viscera to be successfully transplanted in humans. The first fully reported UTx took place from a deceased donor in humans in 2011 in Antalya, Turkey [1] and from a live donor in 2013 in Sweden [2,3].
Why did it take so long?
The uterus is not a vital organ and consequently transplantation could not be attempted until safe and effective immunosuppression was established with vital organ transplants. Moreover, contrary to the other transplanted viscera, the uterus is within the realm of gynecology and not general surgery or urology, the specialties of most abdominal transplant surgeons. Gynecologists typically do not perform transplants.
My interest in these transplants was planted 15 years ago. I was inspired by a young woman who underwent a multivisceral transplant (Liver, Stomach, Pancreas, Intestine) at our program but was still not “whole”: she had a prior hysterectomy and could not bear children…
Would a transplant of the uterus have been possible? How many other patients could benefit from this procedure? I began to look into these questions.
I found out that there are thousands of women of reproductive age who do not have a uterus and suffer from “uterine factor infertility”. In addition to surgical hysterectomies, 1 in 5000 women are born without a uterus (MRKH syndrome). In the US their number is estimated at 50.000. In Greece this number is likely 1500-2000. These are otherwise healthy women who have normal ovaries and a vagina which is usually underdeveloped.
Until now, they could have children only by adoption or surrogacy, two choices that have helped many women fulfill their dreams. Unfortunately, these are not viable options for many women because of personal, cultural, religious, and legal reasons which are prohibitive.
In addition, a uterus transplant is the only option that gives the woman the opportunity to carry the responsibility, pain and joy of bearing and giving birth to her child, a child with her genetic signature.
For these reasons I started exploring the feasibility of UTx.
Looking at existing experimental models, Brannstrom and his associates had shown that uterine transplants are feasible in small animals and could produce healthy offspring [4]. Positive results in small animals may not always be transferable to large animals, let alone humans.
The next question was whether we could reproduce these results in large animals? My associates Drs Akin Tekin, Tom De Faria and Takis Tryphonopoulos, a very committed group of surgeons, worked diligently with me.
In choosing an appropriate large animal model, one has to consider that the reproductive system is species specific. The one closest to humans, anatomically and physiologically, is the reproductive system of the primates.
Experiments with primates are limited by very strict regulations and high cost.
In addition, effective immunosuppression has been problematic and requires extremely high doses which can be toxic [5]. Baboons find creative ways to get rid of the pills, consequently the medications must be administered parenterally. In addition, experiments are emotionally difficult to perform because of the humanoid features of these animals.
Our initial objective was to test whether we could establish, long term, the viability of the laboratory animals bearing healthy uterine grafts. We wanted to study rejections and response to treatment. We started with MHC defined mini-Swine, a model with an established record in Transplantation. Dr David Sachs was kind enough to provide the animals.
All our experiments were done with the preconceived notion that if successful, we planned to use deceased donors in humans. This allowed us the liberal use of donor tissues without limitations related to postoperative donor survival. All donors were euthanized after donation.
We developed a heterotopic model [6]: we used the uterine graft attached to a vaginal cuff en block with their vascular pedicle. The latter included the donor uterine, iliac vessels, the abdominal aorta, the corresponding veins and the inferior vena cava.
The operation was extraperitoneal. The abdominal aorta and inferior vena cava of the donor were anastomosed to the lower abdominal vessels of the recipient, much like a kidney transplant. We exteriorized the donor vagina, just like an ileostomy, in order to have easy access to the graft which could be assessed visually every day. Hysteroscopies and biopsies could be performed with simple sedation (Figure 1).

Figure 1. Heterotopic Uterus Transplantation in Mini Swine. Note that the Uterus bares little resemblance to the human uterus. The donor Aorta and IVC are anastomosed end to the side to the recipient aorta and IVC. The donor vagina is exteriorized for easy access.
Immunosuppression was based on Tacrolimus and Steroids.
The biologic behavior of the uterus proved to be similar to the kidney allografts. We learned that the uterine allografts in the mini swine develop rejections which are reversible with the standard regimens. Long term survival with a healthy uterine allograft was possible!
At that time, we moved to primates housed in the Mannheimer foundation, an outstanding primate facility near Miami.
The baboons are very curious animals. If we exteriorized the vagina, they were certain to chew on it and cause fatal bleeding. For this reason, we performed a hysterectomy and placed the transplant orthotopically. We anastomosed the pedicles of the abdominal aorta and inferior vena cava of the donor to the corresponding vessels of the recipient. The donor vagina was anastomosed to the vagina of the recipient. Follow up of the uterine graft was performed with hysteroscopies which had to take place under general anesthesia [7] (Figure 2).

Figure 2. Uterus transplant in a baboon. 2a: the graft after reperfusion 2b: The graft before closure of the abdomen.
A fortuitous coincidence was that at that time I received an Honorary Degree at the University of Gothenburg and had the opportunity to meet the Swedish team. They were performing uterine autotransplants in baboons [8]. These had to be performed in a WHO approved facility in Nairobi, Kenya because of severe regulatory restrictions in Sweden. They invited me to participate!
I gladly did that and subsequently invited them to work with us at the Mannheimer Foundation.
The Swedish group had a primary focus on living related donors in humans, so the animal transplants in Nairobi were autotransplants and imitated transplants from a living donor.
The Transplants in Florida were allografts: the donor was euthanized after donation, the transplant imitated UTx from a deceased donor.
There are some very important technical differences between the 2 kinds of transplants.
In the autotransplants, the blood inflow is from the Uterine artery and outflow through the Uterine vein. These vessels are very small and thin, particularly in the baboons. They need to be dissected without damage. Anastomoses are very delicate.
In the transplants we performed in Florida, the vascular anastomoses were performed with the more robust aorta and IVC.
All baboons survived the surgery and postoperative course. From the experiments in Florida, only one survived long term with a healthy uterus graft. The others had to be euthanized [n=3] due to weight loss, one lost her graft to rejection due to inadequate immunosuppression.
How are these techniques relevant to human transplantation?
In the human living donor model, the internal iliac artery and its major branches have to be preserved or there is a risk of severe pelvic ischemia. Arterial inflow is through the uterine arteries and venous outflow by the uterine veins. These vessels, particularly the uterine vein, are wrapped around the ureters of the donor and must be freed from the ureters intact. The required dissection is very delicate and is performed with diathermy for the most part. It may result in damage of the ureters directly or indirectly by destroying their blood supply.
In the deceased donor model, the vital organs are removed first. The abdominal aorta, IVC, common and external iliac vessels are included with the vital organs which are recovered contemporaneously and cannot be used for the UTx.
In angiograms we performed ex vivo in resected human uteri, we found that there is a vascular venous plexus within the broad ligament which could be damaged when skeletonized.
As a consequence, the uterine graft is recovered with the internal iliac vessels and their branches en block with the broad ligament.
The donor ureter, distal to its crossing the internal iliac artery (distal 2-3 cm) is included in the uterine graft.
Anastomoses are performed with the robust internal iliac vessels.
Both living and deceased donor uteri are recovered with the (utero)ovarian vessels. The (utero)ovarian vein is used to supplement the venous drainage of the graft if needed. The (utero)ovarian artery has been rarely used. In the living donor, the ovaries have to be carefully preserved.
Besides the technique, there are other major differences between living and deceased donor uterine transplantation.
The supreme advantage of living donation is control of the timing of the operation and the ability to prepare both the donor and recipient for the transplant. This includes possible hormonal preparation of an older donor.
Greatest disadvantage is the risk to the donor. The risk is not exactly known but is expected to be low. Main concerns are the length of the donor operation and the extensive dissection of the pelvic vital structures of the donor, particularly the ureters, as already mentioned.
Supreme disadvantage of the deceased donor UTx is the shortage of female donors of reproductive age. The timing of the transplant cannot be controlled. Supreme advantage is the lack of risk to the donor.
In general, living donors have better long-term patient and graft survival outcomes. This is hardly an advantage in UTx. Contrary to other transplants, the uterine graft is the only known ephemeral graft. It is intended to be removed after the birth of one or two healthy babies.
Simultaneous with our work in the animal lab, we presented our efforts to the Ethics Board of the University of Miami. In collaboration with the Director of the Institute of Bioethics and Health Policy at the University of Miami, Dr Ken Goodman, we held a “Town Hall” meeting. Faculty and students of the University of Miami as well as interested members of the community and clergy were invited and we participated in lively discussions regarding the propriety of attempting Uterus Transplantations in humans.
Main concerns were related to the unknown risk of performing a non-vital transplant for a healthy person, the risk to the fetus and in case of living donation to the donor.
A mitigating factor was that the healthy recipient is not subjected to lifelong immunosuppression. The immunosuppression is stopped when the graft is removed and not given for life.
The outcome of the “Town Hall” meeting was positive overall, although, as expected the scrutiny was intense.
In the meantime, the Swedish team was ready to proceed with a human trial and invited me to participate. We performed 9 uterine transplants from living donors [2]. It was exactly at that time that I relocated from the University of Miami to the Cleveland Clinic.
It was also the beginning of a very close collaboration with the Department of Gynecology and Obstetrics at the Cleveland Clinic, one of the best in the World headed by Dr Tommaso Falcone.
I participated in all the Swedish cases, Dr Falcone joined me and with his teammate, Dr Rebecca Flyckt became enthusiastic partners.
Dr Falcone and I were impressed that even in the very experienced hands of the Swedish team, the donor operation took 8-10 hours including the very extensive dissection of vital structures, particularly the donor ureters.
For these reasons, at the Cleveland Clinic we decided to continue our focus in deceased donor transplants. The very elaborate process of Review by the Institutional review board had to be repeated. We obtained approval after 2 years of deliberations.
After several practice runs, we developed a simple method to perform the deceased donor hysterectomy safely [9]. It starts with a new “arrow shaped” incision which facilitates the pelvic dissection and expedites the organ recovery. It can be accomplished in 1.5 hrs (Figure 3, 4).

Figure 3. The deceased donor. Human UTx: a: Donor Incision, b: Vascular pedicles, c: Uterus in place.

Figure 4 a: Uterine graft from deceased donor, 4b: ex vivo angiogram.
The first live birth from a live uterus donor was done in 2014 in Sweden [2]. It was followed in the same year by the first Uterus transplant in the US by a deceased donor which took place at the Cleveland Clinic [11]. Unfortunately, the graft failed because of an arterial anastomotic disruption of the left arterial anastomosis (Uterine to external iliac artery), due to candida endarteritis. This was a life- threatening complication. Thankfully, the patient recovered completely, but this serious complication necessitated an extensive, time-consuming review of the protocols as well as ethical review.
There has been some confusion about the “firsts”…
The definition of a successful uterine transplant is one that results in the birth of a healthy offspring.
The Turkish case [1], although technically successful was not recognized as “successful” till 2020 when the recipient delivered a healthy baby after multiple, heroic and protracted attempts.
In the meantime, the pioneering Swedish trial [2], in addition to the technical success, produced the first live birth followed by a series of more healthy babies. It proved, in a fairly short time, both the feasibility and efficacy of this operation.
The success of the Swedish trial was undoubtedly the most influential factor in the development of Uterine Transplantation worldwide. It encouraged new investigators to proceed using living donors, for the most part. It showed that UTx, although not vital, can nurture life and deliver it safely into this world.
The first live birth from a deceased donor took place in Brazil in 2017 [10]. It was followed by the first delivery of a healthy child from a deceased donor in the US at the Cleveland Clinic in a few months [12]. They were both momentous events showing that UTx from a deceased donor may be just as effective as the living donors.
Unfortunately, our reservations about living donation have been substantiated in the initial world experience. Every busy living donor program has seen at least one ureteral complication which required a surgical or endoscopic correction. No doubt, more experience will help optimize the techniques and further improve the results.
In the meantime, more successful UTxs with deceased donors, including the ones in Cleveland, have been reported invigorating the interest in deceased donors.
We have since performed additional 7 cases with one failure. There have been 4 healthy babies from our patients, the largest series to this day. We are in the process of in vitro fertilization for the remaining 2 (Figure 5, 6).

Figure 5. Doppler Ultrasound of the fetus midterm from our First Successful UTx.
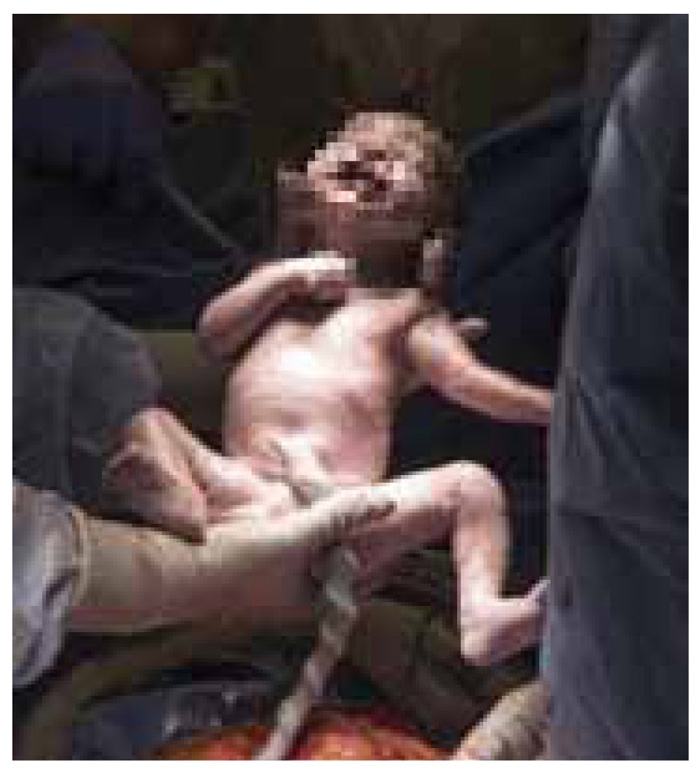
Figure 6: The birth of the first baby in the US from a deceased donor: a magical moment.
An International Registry of UTx is in the making. In the meantime, reports on worldwide experience are based on presentations at National and International Meetings.
It is estimated that there have been 60 UTxs performed worldwide with technical success in 90% of them. There have been more than 30 babies, all healthy. More babies are forthcoming as efforts for successful pregnancies continue in the technically successful cases.
Thus far, all uterine transplants have been financed by Institutional Grants and Private donations with the exception of one UTx from a living donor which has been “self-financed”. Wide acceptance will result in more extramural funding which will help the dissemination of the procedure.
The journey to UTx continues, but is no longer in uncharted waters. We landed in new territory, one that seems to be more fertile with each passing day.
Conflict of interest disclosure
None to declare
Declaration of funding sources
None to declare
Author contribution
Andreas G Tzakis: conception, writing, data interpretation and review of the final draft of the article.
References
1. Ozkan O, Dogan NU, Ozkan O, Mendilcioglu I, Dogan S, Aydinuraz B, et al. Uterus transplantation: From animal models through the first heart beating pregnancy to the first human live birth. Womens Health (Lond). 2016;12(4):442-9.
2. Brännström M, Johannesson L, Dahm-Kähler P, Enskog A, Mölne J, Kvarnström N, et al. First clinical uterus transplantation trial: a six-month report. Fertil Steril. 2014;101(5):1228-36.
3. Brännström M, Johannesson L, Bokström H, Kvarnström N, Mölne J, Dahm-Kähler P, et al. Livebirth after uterus transplantation. Lancet. 2015;385(9968):607-16.
4. Díaz-García C, Johannesson L, Shao R, Bilig H, Brännström M. Pregnancy after allogeneic uterus transplantation in the rat: perinatal outcome and growth trajectory. Fertil Steril. 2014;102(6):1545-52.e1.
5. Thiru S, Collier DS, Calne R. Pathological studies in canine and baboon renal allograft recipients immunosuppressed with FK-506. Transplant Proc. 1987;19(5 Suppl 6):98-9.
6. Avison DL, DeFaria W, Tryphonopoulos P, Tekin A, Attia GR, Takahashi H, et al. Heterotopic uterus transplantation in a swine model. Transplantation. 2009;88(4):465-9.
7. Tryphonopoulos P, Tzakis AG, Tekin A, Johannesson L, Rivas K, Morales PR, et al. Allogeneic uterus transplantation in baboons: surgical technique and challenges to long-term graft survival. Transplantation. 2014;98(5):e51-6.
8. Enskog A, Johannesson L, Chai DC, Dahm-Kähler P, Marcickiewicz J, Nyachieo A, et al. Uterus transplantation in the baboon: methodology and long-term function after auto-transplantation. Hum Reprod. 2010;25(8):1980-7.
9. Tzakis A, Olausson M, Falcone T. Surgical Technique of Deceased Donor in Uterus Transplantation. In: Brännström M. (eds) Uterus Transplantation. Springer, Cham. 2020;119-27.
10. Ejzenberg D, Andraus W, Baratelli Carelli Mendes LR, Ducatti L, Song A, Tanigawa R, et al. Livebirth after uterus transplantation from a deceased donor in a recipient with uterine infertility. Lancet. 2019;392(10165):2697-704.
11. Flyckt R, Kotlyar A, Arian S, Eghtesad B, Falcone T, Tzakis A. Deceased donor uterine transplantation. Fertil Steril. 2017;107(3):e13.
12. Flyckt R, Falcone T, Quintini C, Perni U, Eghtesad B, Richards EG, et al. First birth from a deceased donor uterus in the United States: from severe graft rejection to successful cesarean delivery. Am J Obstet Gynecol. 2020;223(2):143-51.