ACHAIKI IATRIKI | 2025; 44(Suppl 1):46–54
Review
Nikolaos Polychronopoulos*, Eirini Kechagia*, Dimitrios Velissaris, Christos Michailides
Department of Internal Medicine, University Hospital of Patras, Greece
*Equal contribution
Received: 26 Feb 2025; Accepted: 28 Apr 2025
Corresponding author: Christos Michailides, MD, MSc, Greece, Tel.: +30 6982086050, e-mail: up1047708@ac.upatras.gr, ORCID: 0000-0003-1712-9815
Keywords: Biomarkers, infections, sepsis, prognosis, diagnosis, pancreatic stone protein
Abstract
Sepsis is a life-threatening condition caused by an abnormal immune response to an infection, leading to severe multi-organ failure and significantly high mortality rates. It remains a challenge for daily clinical practice, as it requires early diagnosis and differentiation of patients, along with personalized treatment strategies, in order to achieve optimal clinical outcomes and eliminate morbidity.
Inflammatory biomarkers play a crucial role in the early detection of sepsis, providing valuable insights into disease progression, monitoring the patient’s response to treatment and guiding medical interventions. Procalcitonin (PCT) and C-reactive protein (CRP) are conventional infection biomarkers that are commonly utilized to differentiate a bacterial infection from a non-bacterial infection, although their diagnostic accuracy remains limited.
Emerging biomarkers, including Presepsin (PSEP), Pancreatic Stone Protein (PSP), and Myxovirus Resistance Protein A (MxA), demonstrate higher sensitivity and specificity, providing more precise information for diagnostic accuracy and prognosis. PSP levels rise earlier than CRP and PCT, remaining more stable throughout the hospitalization, whereas MxA effectively distinguishes viral from bacterial infections. Moreover, Serum lactate, neutrophil-to-lymphocyte ratio (NLR), and ferritin are essential for evaluating the infection’s evolution and are frequently incorporated into screening protocols, management guidelines, and sepsis definitions. PCT-guided antibiotic treatment reduces antimicrobial consumption by limiting unnecessary or prolonged treatments without jeopardizing safety. Despite the potential of biomarkers, no single biomarker has sufficient sensitivity and specificity to confirm or exclude sepsis, monitor its progression, or guide treatment decisions.
Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. In patients with hyper-inflammatory immune response syndrome, early recognition, risk stratification and personalized medical interventions play a crucial role in preventing life-threatening organ dysfunction [2]. Although in recent years basic science studies have been conducted to determine the mechanisms of sepsis leading to organ failure, it remains a challenge for clinicians due to the phenotypic heterogeneity and the increased mortality rates.
A biomarker is defined as any measurable molecule that can distinguish a normal from a pathological condition or provide valuable information to guide therapeutic interventions. The ideal biomarker should provide a rapid result with excellent diagnostic performance, sensitivity, specificity, repeatability, cost-effectiveness and ease of interpretation [3]. Over the last few decades, a wide range of infection and sepsis biomarkers have been used providing the opportunity for an early diagnosis and more specific therapeutic decisions, which can optimize recovery and minimize unnecessary usage of antibiotics such as with PCT and CRP [4].
This narrative review aims to provide insight into the role of biomarkers in early sepsis diagnosis, prognosis, and the optimization of therapeutic interventions (Figure 1).
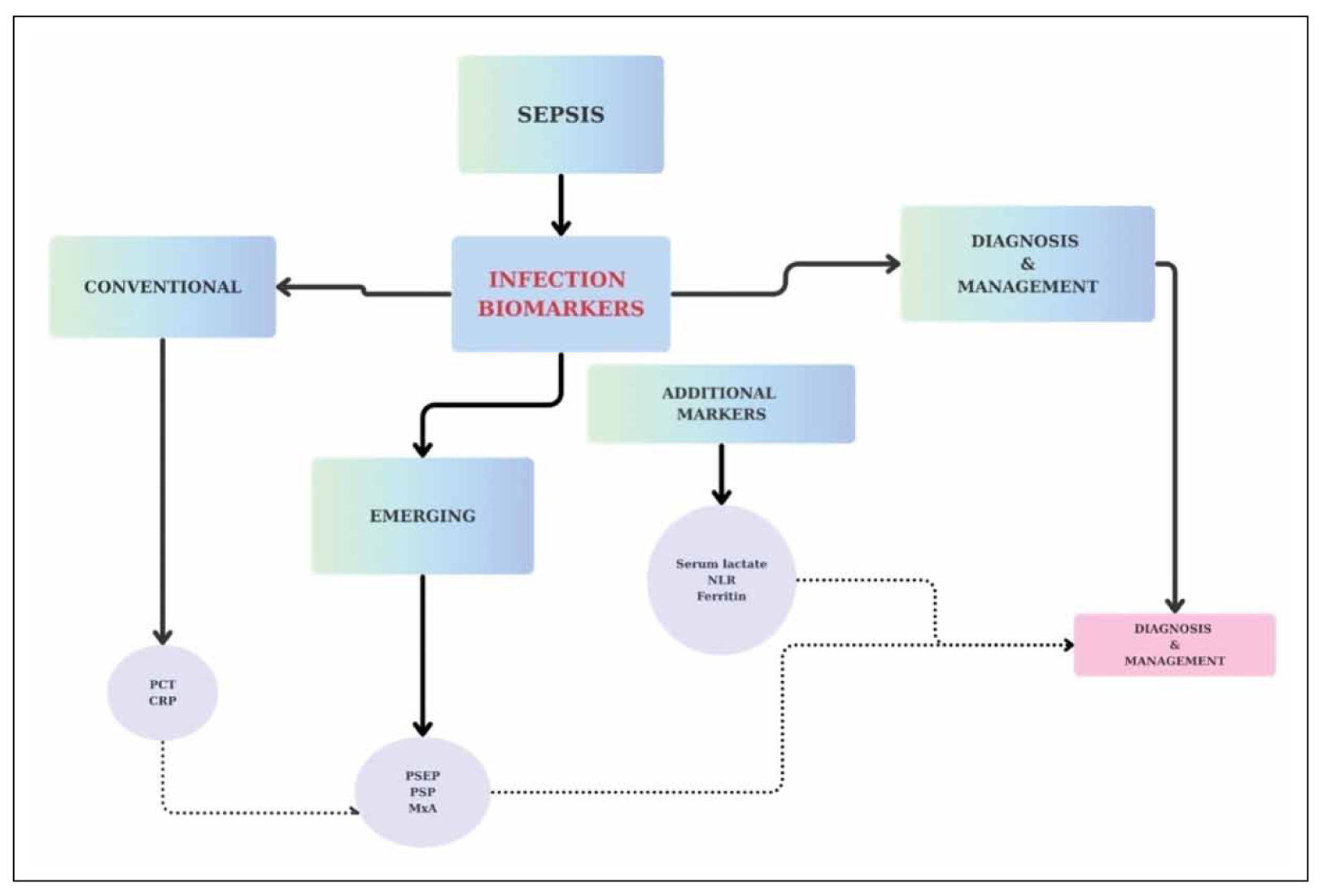
Figure 1. Infection biomarkers.
Biomarkers and diagnosis (rule in)
The indispensable role of biomarkers, including PCT and CRP, has been unequivocally recognized as a fundamental aspect of contemporary clinical practice. Notably, PCT, alongside CRP, can adequately differentiate between bacterial and non-bacterial infections as well as distinguish patients with SIRS from sepsis [5,6]. However, the diagnostic accuracy of each biomarker alone cannot provide a certain diagnosis [4,6]. Hence, clinical scores and combinations with other biomarkers have emerged to improve their diagnostic performance [4,7]. Although PCT is widely regarded as superior to CRP, it is not a conclusive diagnostic test for sepsis because PCT levels can also be significantly elevated in various other pathological conditions, particularly in patients with compromised renal function, thus complicating the identification of an underlying infection [8,9]. Moreover, recent studies have established PSEP as an important biomarker for sepsis, due to its early elevation and substantially increased levels in gram-negative bacterial infection [10]. Furthermore, it demonstrates high specificity (82.6%) for sepsis diagnosis, with an AUC of 0.87. According to a recent study, PSEP had a good diagnostic value for sepsis, with a summary sensitivity of 0.805 (0.759-0.844), establishing it as a promising tool in the emergency department [10]. Contrary to other biomarkers of sepsis, PSEP has the capacity to differentiate septic shock from sepsis, providing more specific diagnostic insight [11]. Nevertheless, PSEP demonstrates only moderate diagnostic accuracy in differentiating sepsis from non-sepsis cases and should be utilized in conjunction with other biomarkers and clinical scoring systems to definitively confirm the diagnosis of infection [12]. Additionally, PSP represents a promising point-of-care biomarker for sepsis, distinguished by its ability to rise in blood levels five days earlier than conventional biomarkers like PCT and CRP [13]. Notably, PSP is considered a more reliable biomarker for sepsis because its serum levels remain stable after burn injury and subsequent debridement, in contrast to CRP and PCT, which increase significantly following inflammatory or surgical events [14]. PSP demonstrates an overall sensitivity of 0.88 and specificity of 0.78 for sepsis diagnosis with an AUC value of 0.90 [14]. In addition, the detection of Aspergillus, specifically the invasive fungal infection, remains a formidable challenge for the medical field because definitive confirmation is often delayed and reliant on invasive procedures [15,16]. In this regard, serum galactomannan antigen levels can contribute to the early and accurate diagnosis of candidemia, demonstrating a sensitivity of 0.71 (0.64-0.78) and a high specificity of 0.89 (0.89-0.92) in immunocompromised patients [15,16]. Consequently, the detection of galactomannan antigen levels has become increasingly prominent in the diagnostic process for this infection, providing a valuable tool for early identification, which plays a pivotal role in enhancing clinical outcomes and reducing both mortality and morbidity rates. Diagnostic biomarkers are summarized in Table 1 and Figure 2.
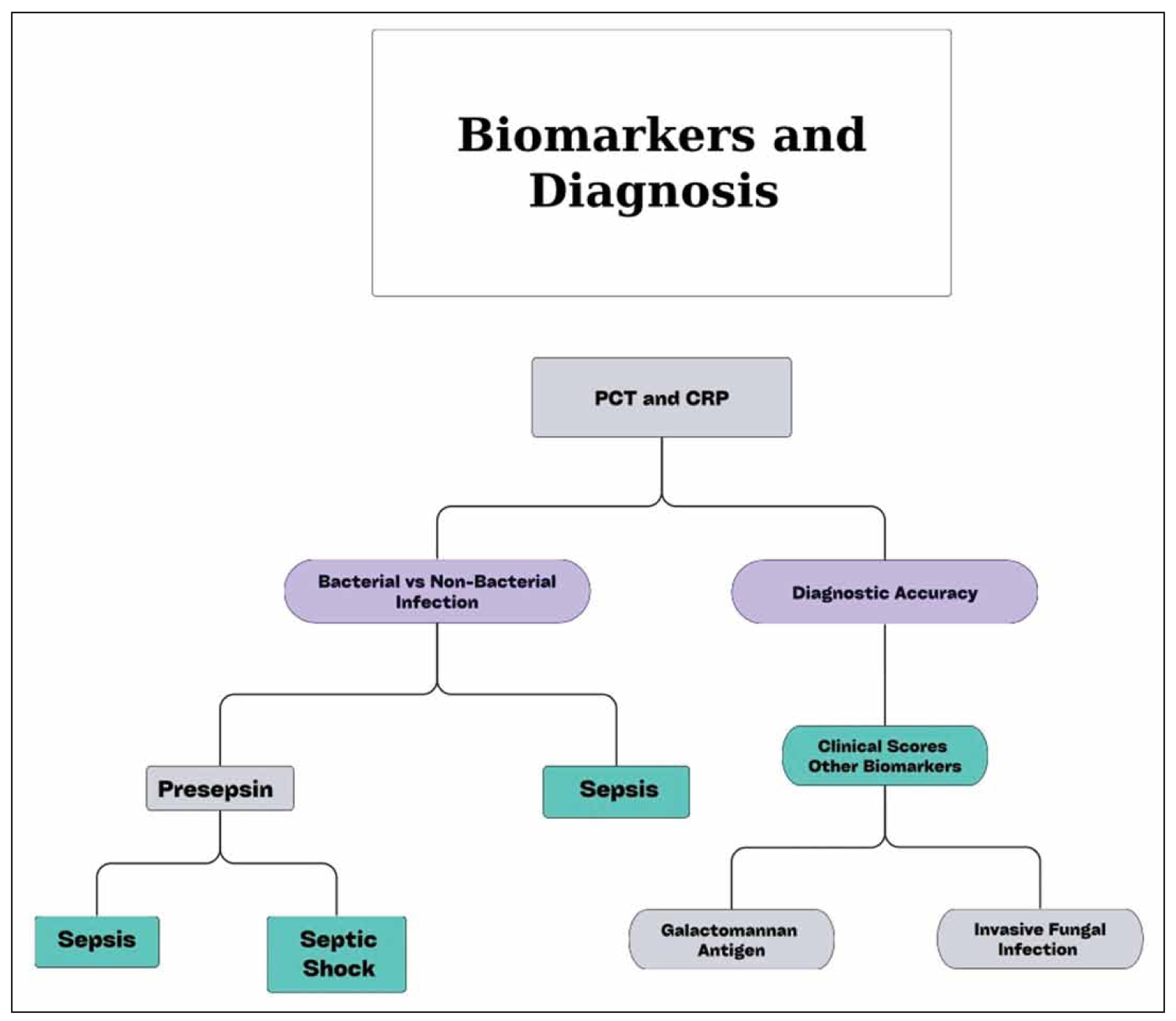
Figure 2. Diagnostic biomarkers and their clinical utility.
Biomarkers and exclusion (rule out)
Septic patients exhibit a wide range of nonspecific clinical manifestations, thereby complicating the differentiation from other serious medical conditions, potentially leading to misdiagnosis. Therefore, infection biomarkers play a crucial role in reducing diagnostic uncertainties by excluding non-relevant patients. The most widely utilized inflammatory biomarker in routine clinical practice, CRP, is not considered adequate for distinguishing infection from non-infection in patients, as its levels can be elevated in a wide range of non-infection inflammatory pathological conditions such as malignancy, trauma, and autoimmune diseases. Thus, CRP alone, due to its limited specificity and negative prognostic value, is not capable of ruling out sepsis [17,18]. However, an interesting finding from research is that the combination of PSP and CRP is significantly more reliable for ruling out infection in hospitalized patients, demonstrating increased effectiveness compared to the combination of PSP with PCT. Therefore, the integration of PSP with CRP can enhance the diagnostic accuracy of infections, leading to an increase in AUC from 0.81 to 0.90, while concurrently improving the sensitivity of PSP without compromising its specificity [19]. Specifically, the combined use of PSP and CRP demonstrates a high sensitivity of 0.81 with an ROC AUC of 0.90, which is notably better than CRP alone [19]. However, it is crucial to highlight that PSP alone has a high positive predictive value of 0.85 for the detection of infection and its levels exhibit a significantly clearer distinction between infected and non-infected patients, with limited overlap [19]. Commonly used inflammatory biomarkers in daily medical practice, such as CRP and PCT, lack the specificity required to reliably differentiate between bacterial and viral infections. In contrast, MxA has emerged as a valuable biomarker for accurate exclusion of viral infections, as its levels are significantly higher in patients with viral infections (83.3) compared to those with bacterial infections (33.8) with high sensitivity [20,21]. It is important to highlight that the expression of MxA in monocytes did not demonstrate a
statistically significant reduction in patients with viral-bacterial co-infection when compared to those with purely viral infection. This overlap complicates the distinction between viral infections and co-infections, making it more challenging to reliably rule out the presence of bacterial involvement [20,21]. Excluding biomarkers are summarized in Table 2.
Biomarkers and monitoring
Monitoring sepsis patients is a significant challenge in the medical field due to the nonspecific symptoms and the critical role of timing in disease progression. Biomarkers are essential in assessing the infection’s evolution and guiding clinical decisions. The measurement of CRP is widely utilized for diagnosing and, more importantly, for monitoring the progression of inflammatory conditions, particularly sepsis [22]. Due to its rapid elevation in response to inflammation and its prompt decline upon resolution, CRP serves as a valuable biomarker for assessing therapeutic efficacy [23]. In septic patients, CRP levels peaked on day 2 before gradually declining until day 10, whereas in septic shock, they continued to rise throughout the ICU stay, reaching a median of 179 (66–225) mg/L by day 10 [24]. It is worth noting that an increase in CRP after 48 hours, particularly when exceeding 100 mg/L, was associated with more significant organ dysfunction, prolonged ICU stays, and an elevated risk of all-cause mortality [24].Additionally, sepsis significantly alters white blood cell populations, driving its pathophysiology and immunoparalysis [25]. An initial surge in neutrophils and monocytes transitions rapidly to lymphopenia, characterized by extensive apoptosis of B cells, CD4+ T cells, and CD8+ T cells [25]. An eosinophil count < 0.05 x 10^9/L after 96 hours in the ICU is a moderate predictor of 30-day mortality and is associated with prolonged ICU and hospital stays [26]. However, in sepsis, the white blood cell (WBC) count may serve as a marker but it’s unreliable due to the variability in the septic response and its potential overlap with conditions such as burns and other inflammatory diseases, which may also influence WBC levels [27]. Another promising sepsis monitoring biomarker is NLR. An increased NLR can occur in various conditions such as bacterial or fungal infections, acute stroke, myocardial infarction, severe trauma, post-surgical complications, and any condition involving tissue damage that triggers SIRS because it suppresses neutrophil apoptosis, enhancing neutrophil-mediated killing in the innate immune response [28,29]. Therefore, NLR has been established as a reliable biomarker for the diagnosis and monitoring of sepsis. Notably, NLR levels in critically ill septic patients with poor prognosis and high mortality rates are significantly elevated compared to those observed in patients who exhibit a well-regulated immune response, characterized by a balanced activation of both innate and adaptive immune mechanisms. Furthermore, serum lactate levels are widely recognized as a marker of tissue hypoxia and are frequently incorporated into screening protocols, management guidelines, sepsis definitions (persistent serum levels >2mmol/L), and therapeutic decisions [30,31]. Although blood lactate levels change gradually and should not be the sole guide for therapy, serial measurements can help assess the patient’s trajectory and prompt a reassessment of therapy if levels remain stable or rise [32]. Biomarkers that are used for monitoring are summarized in Table 3.
Biomarkers and prognosis
WBC as a biomarker plays a pivotal role in both sepsis and survival outcomes. More specifically, T-cells have shown strong prognostic value in ICU patients with sepsis, with low T-cell levels being associated with increased risk of mortality. ICU patients with T-cell counts exceeding 0.36 per nL exhibited nearly double the survival rate compared to those with lower counts [33]. It is worth noting that basophils serve as a superior prognostic predictor compared to other immune cells because their fluctuating levels during the ICU stay effectively predict 28-day mortality, with low concentrations associated with adverse outcome, highlighting the prognostic value of WBC in sepsis [25,34]. Moreover, continuous monitoring of CRP in septic patients, particularly during the initial five days of antibiotic treatment, can assist in assessing prognosis and predicting outcomes [35]. Specifically, a significant CRP reduction was observed in survivors compared to non-survivors, with a decrease of 0.31 or more post-antibiotic initiation, indicating a favorable prognosis [36]. Thus, a substantial decrease in serum CRP levels often signifies the resolution of sepsis, providing valuable insights into the prognostic value of CRP in sepsis-related mortality [35,36]. Furthermore, a ferritin level of ≥591.5 ng/ml was identified as an independent predictor of in-hospital mortality in septic patients, with serum concentrations exceeding this threshold being associated with a 119% increase in the risk of in-hospital mortality [37]. Studies indicate that elevated ferritin levels after 24 hours are strongly associated with prolonged ICU length of stay (LOS) and hemophagocytosis, a process that may occur in sepsis [38]. Specifically, ferritin levels exceeding 2000 µg/L have been identified as a mortality predictor, also linked with disease severity in hospitalized patients [39]. Additionally, high concentrations of the septic biomarker presepsin are directly correlated with elevated mortality rates and a significant risk of severe complications, including acute kidney injury (AKI), septic shock, ARDS, and DIC. Elevated presepsin levels at hospital admission are strongly associated with 60-day in-hospital mortality [40].Consequently, increased presepsin at ICU admission and on day 2 was predictive of AKI and the development of DIC, while presepsin levels measured on days 1-3, in conjunction with the Glasgow prognostic score, effectively predicted the onset of ARDS [41,12]. PSP demonstrated a significant interaction between time and sepsis presence, with a steeper increase in septic patients (interaction P < 0.001). The median PSP on admission was 162 ng/dL in sepsis patients and 74.5 ng/dL in non-septic patients, tripling within 72 hours and doubling within 48 hours before sepsis became clinically evident [42]. Furthermore, in a prospective observational cohort study, PSP on admission was significantly higher in patients who were readmitted, with a median value of 203 ng/dL compared to 71 ng/dL in those who were not [43,44]. PSP is also strongly associated with both ICU mortality and the combined endpoint of sepsis/septic shock. With high sensitivity (>85%), PSP is a valuable tool for clinicians in determining hospitalization and intensive care needs, as its levels can guide decisions on discharge or hospitalization following an IAI diagnosis [44,13]. Prognostic biomarkers are summarized in Table 4.
Biomarkers and therapeutic treatment
A significant public health concern is the excessive use of non-specific antibiotics in patients with infections of varying severity in terms of morbidity and clinical condition, as it leads to the development of resistant strains and the emergence of complications. The use of PCT-guided antibiotic therapy safely reduces antimicrobial consumption by limiting unnecessary or prolonged treatments. In ICU patients, PCT-guided therapy, including antibiotic cessation rules, significantly shortened antibiotic duration [45]. Studies on respiratory tract infections and peritonitis have shown reduced antibiotic use without adverse outcomes. More specifically, in peritonitis patients, antibiotic prescriptions were 72% lower with PCT, and the duration was one day shorter, with no difference in infection recurrence. PCT guidance has been effective in reducing antibiotic use and improving outcomes in a wide range of infections, including respiratory tract infections and sepsis [46]. By using PCT cut-offs, antibiotics can be withheld or discontinued when there is a level drop, reducing exposure and side effects while maintaining safety. This approach demonstrates lower mortality and fewer complications than standard therapy, emphasizing PCT’s value in optimizing antibiotic use [47]. Last but not least, SuPAR is a key biomarker for assessing the risk of progression to severe respiratory failure in COVID-19 pneumonia patients, with levels exceeding 6 ng/mL. Early identification using SuPAR, in combination with the qSOFA score, enhances prognostic accuracy and enables timely therapeutic intervention [48]. When SuPAR levels are elevated, the likelihood of severe respiratory failure increases, but this risk can be significantly mitigated with the administration of anakinra, an interleukin-1 inhibitor, for 10 days based on suPAR levels. This approach emphasizes the critical role of suPAR as a therapeutic guide, aiding in the early management of COVID-19 progression and improving patient outcomes [49]. Biomarkers that guide treatment are summarized in Table 5.
Conclusion
Sepsis is a life-threatening condition that remains a persistent challenge for the medical field. The nonspecific and heterogeneous symptoms, combined with the lack of highly sensitive and specific diagnostic tools, substantially increase the risk of misdiagnosis. Septic biomarkers play a fundamental role in enabling early and accurate diagnosis, minimizing the indiscriminate use of antibiotics, and optimizing clinical outcomes by reducing morbidity and mortality rates. Despite the promising potential of biomarkers in sepsis, up until 2025, no individual biomarker demonstrates adequate sensitivity and specificity to rule in or rule out sepsis, monitor the progression of the condition or determine appropriate treatment decisions. Thus, due to the complexity of sepsis, the combination of various biomarkers is required to enhance medical management and capture the diverse aspects of the immunological response. In conclusion, there is an increasing demand for further research and meta-analysis of both promising emerging septic biomarkers and those that have not been sufficiently studied, to provide more specific results, reduce existing limitations, and facilitate the advancement of more personalized and targeted therapies (Figure 3).
Figure 3. Summary of biomarkers’ effectiveness in sepsis.
Conflict of interest disclosure
None to declare.
Declaration of funding sources
None to declare
Author contributions
NP and EK did literature search and wrote the paper; DV edited and supervised the paper; CM designed, wrote and supervised the paper.
References
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-10.
- Komorowski M, Green A, Tatham KC, Seymour C, Antcliffe D. Sepsis biomarkers and diagnostic tools with a focus on machine learning. EBioMedicine. 2022;86:104394.
- Barichello T, Generoso JS, Singer M, Dal-Pizzol F. Biomarkers for sepsis: more than just fever and leukocytosis-a narrative review. CritCare. 2022;26(1):14.
- Pierrakos C, Velissaris D, Bisdorff M, Marshall JC, Vincent JL. Biomarkers of sepsis: time for a reappraisal. Crit Care. 2020;24(1):287.
- Arbutina DD, Milic L, Cuk VV, Juloski JT, Radulovic R, Starcevic A, et al. Significance of Biomarkers in Early Diagnosis of Abdominal Sepsis. Chirurgia (Bucur). 2022;117(1):30-6.
- Schuetz P. How to best use procalcitonin to diagnose infections and manage antibiotic treatment. Clin Chem Lab Med. 2023;61(5):822-8.
- Zaki HA, Bensliman S, Bashir K, Iftikhar H, Fayed MH, Salem W, et al. Accuracy of procalcitonin for diagnosing sepsis in adult patients admitted to the emergency department: a systematic review and meta-analysis. Syst Rev. 2024;13(1):37.
- Mobed A, Darvishi M, Tahavvori A, Alipourfard I, Kohansal F, Ghazi F, et al. Nanobiosensors for procalcitonin (PCT) analysis. J Clin Lab Anal. 2024;38(3):e25006.
- Paudel R, Dogra P, Montgomery-Yates AA, Coz Yataco A. Procalcitonin: A promising tool or just another overhyped test. Int J Med Sci. 2020;17(3):332-7.
- Paraskevas T, Chourpiliadi C, Demiri S, Micahilides C, Karanikolas E, Lagadinou M, et al. Presepsin in the diagnosis of sepsis. Clin Chim Acta. 2023;550:117588.
- Aliu-Bejta A, Atelj A, Kurshumliu M, Dreshaj S, Baršić B. Presepsin values as markers of severity of sepsis. Int J Infect Dis. 2020;95:1-7.
- Velissaris D, Zareifopoulos N, Karamouzos V, Karanikolas E, Pierrakos C, Koniari I, et al. Presepsin as a Diagnostic and Prognostic Biomarker in Sepsis. Cureus. 2021;13(5):e15019.
- Michailides C, Paraskevas T, Demiri S, Chourpiliadi C, Papantoniou K, Aggeletopoulou I, et al. Diagnostic and Prognostic Ability of Pancreatic Stone Protein: A Scoping Review. Int J Mol Sci. 2024;25(11):6046.
- Fidalgo P, Nora D, Coelho L, Povoa P. Pancreatic Stone Protein: Review of a New Biomarker in Sepsis. J Clin Med. 2022;11(4):1085.
- Haydour Q, Hage CA, Carmona EM, Epelbaum O, Evans SE, Gabe LM, et al. Diagnosis of Fungal Infections. A Systematic Review and Meta-Analysis Supporting American Thoracic Society Practice Guideline. Ann Am Thorac Soc. 2019;16(9):1179-88.
- de Oliveira VF, Silva GD, Taborda M, Levin AS, Magri MMC. Systematic review and meta-analysis of galactomannan antigen testing in serum and bronchoalveolar lavage for the diagnosis of chronic pulmonary aspergillosis: defining a cutoff. Eur J Clin Microbiol Infect Dis. 2023;42(9):1047-54.
- Ozger HS, Senol E. Use of infection biomarkers in the emergency department. Turk J Emerg Med. 2022;22(4):169-76.
- Nehring SM, Goyal A, Patel BC. C reactive protein. Stat Pearls [Internet]. TreasureIsland (FL): Stat Pearls Publishing; 2023 Jul 10 [cited 2025 Jan].
- Prazak J, Irincheeva I, Llewelyn MJ, Stolz D, García de Guadiana Romualdo L, Graf R, et al. Accuracy of pancreatic stone protein for the diagnosis of infection in hospitalized adults: a systematic review and individual patient level meta-analysis. Crit Care. 2021;25(1):182.
- Metz M, Gualdoni GA, Winkler HM, Warenits AM, Stöckl J, Burgmann H, et al. MxA for differentiating viral and bacterial infections in adults: a prospective, exploratory study. Infection. 2023;51(5):1329-37.
- Brendish NJ, Davis C, Chapman ME, Borca F, Waddington D, Hill C, et al. Emergency Department point-of-care antiviral host response testing is accurate during periods of multiple respiratory virus co-circulation. J Infect. 2024;88(1):41-7.
- Plebani M. Why C-reactive protein is one of the most requested tests in clinical laboratories? Clin Chem Lab Med. 2023;61(9):1540-5.
- Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32(4):274-8.
- Schupp T, Weidner K, Rusnak J, Jawhar S, Forner J, Dulatahu F, et al. C-reactive protein and procalcitonin during course of sepsis and septic shock. Ir J Med Sci. 2024;193(1):457-68.
- Martin MD, Badovinac VP, Griffith TS. CD4 T Cell Responses and the Sepsis-Induced Immunoparalysis State. Front Immunol. 2020;11:1364.
- Al Duhailib Z, Farooqi M, Piticaru J, Alhazzani W, Nair P. The role of eosinophils in sepsis and acute respiratory distress syndrome: a scoping review. Can J Anaesth. 2021;68(5):715-26.
- Barati M, Alinejad F, Bahar MA, Tabrisi MS, Shamshiri AR, Bodouhi NO, et al. Comparison of WBC, ESR, CRP and PCT serum levels in septic and non-septic burn cases. Burns. 2008;34(6):770-4.
- Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int J Mol Sci. 2022;23(7):3636.
- Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am J Emerg Med. 2020;38(3):641-7.
- Vincent JL, Bakker J. Blood lactate levels in sepsis: in 8 questions. Curr Opin Crit Care. 2021;27(3):298-302.
- Nolt B, Tu F, Wang X, Ha T, Winter R, Williams DL, et al. Lactate and Immunosuppression in Sepsis. Shock. 2018;49(2):120-5.
- Weinberger J, Klompas M, Rhee C. What Is the Utility of Measuring Lactate Levels in Patients with Sepsis and Septic Shock. Semin Respir Crit Care Med. 2021;42(5):650-61.
- Hohlstein P, Gussen H, Bartneck M, Warzecha KT, Roderburg C, Buendgens L, et al. Prognostic relevance of altered lymphocyte subpopulations in critical illness and sepsis. J Clin Med. 2019;8(3):353.
- Chen X, Zhu X, Zhuo H, Lin J, Lin X. Basophils absence predicts poor prognosis and indicates immunosuppression of patients in intensive care units. Sci Rep. 2023;13(1):18533.
- Anush MM, Ashok VK, Sarma RI, Pillai SK. Role of C-reactive Protein as an Indicator for Determining the Outcome of Sepsis. Indian J Crit Care Med. 2019;23(1):11-4.
- Koozi H, Lengquist M, Frigyesi A. C-reactive protein as a prognostic factor in intensive care admissions for sepsis: A Swedish multicenter study. Int Care Med. 2020;46(1):73-79
- Fang YP, Zhang HJ, Guo Z, Ren CH, Zhang YF, Liu Q, et al. Effect of Serum Ferritin on the Prognosis of Patients with Sepsis: Data from the MIMIC-IV Database. Emerg Med Int. 2022;2022:2104755.
- Gunasekaran C, Eastwood GM, Peck L, Young H, Serpa Neto A, Bellomo R. Evaluation of ferritin and the ferritin index as prognostic biomarkers in septic shock. Aust Crit Care. 2023;36(5):723-31.
- He L, Guo C, Su Y, Ding N. The relationship between serum ferritin level and clinical outcomes in sepsis based on a large public database. Sci Rep.2023 29;13(1):8677.
- Pizzolato E, Ulla M, Galluzzo C, Lucchiari M, Manetta T, Lupia E, et al. Role of presepsin for the evaluation of sepsis in the emergency department. Clin Chem Lab Med. 2014;52(10):1395-400.
- Yang HS, Hur M, Yi A, Kim H, Lee S, Kim SN. Prognostic value of presepsin in adult patients with sepsis: Systematic review and meta-analysis. PLoS One. 2018;13(1):e0191486.
- Klein HJ, Niggemann P, Buehler PK, Lehner F, Schweizer R, Rittirsch D, et al. Pancreatic Stone Protein Predicts Sepsis in Severely Burned Patients Irrespective of Trauma Severity: A Monocentric Observational Study. Ann Surg. 2021;274(6):e1179-e1186.
- Zuercher P, Moser A, Garcia de Guadiana-Romualdo L, Llewelyn MJ, Graf R, Reding T, et al. Discriminative performance of pancreatic stone protein in predicting ICU mortality and infection severity in adult patients with infection: a systematic review and individual patient level meta-analysis. Infection. 2023;51(6):1797-807.
- Michailides C, Lagadinou M, Paraskevas T, Papantoniou K, Kavvousanos M, Vasileiou A, et al. The Role of the Pancreatic Stone Protein in Predicting Intra-Abdominal Infection-Related Complications: A Prospective Observational Single-Center Cohort Study. Microorganisms. 2023;11(10):2579.
- Papp M, Kiss N, Baka M, Trásy D, Zubek L, Fehérvári P, et al. Procalcitonin-guided antibiotic therapy may shorten length of treatment and may improve survival-a systematic review and meta-analysis. Crit Care. 2023;27(1):394.
- Briel M, Schuetz P, Mueller B, Young J, Schild U, Nusbaumer C, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168(18):2000-7; discussion 2007-8.
- Schuetz P. How to best use procalcitonin to diagnose infections and manage antibiotic treatment. Clin Chem Lab Med. 2023;61(5):822-8.
- Infantino M, Morena L, Di Pietro MA, Romanin B, Cimolato B, Rocca BAL, et al. Soluble urokinase Plasminogen Activator Receptor (suPAR) levels are predictive of COVID-19 severity: an Italian experience. Clin Immunol. 2022;242:109091.
- Adami ME, Kotsaki A, Antonakos N, Giannitsioti E, Chalvatzis S, Saridaki M, et al. qSOFA combined with suPAR for early risk detection and guidance of antibiotic treatment in the emergency department: a randomized controlled trial. Crit Care. 2024;28(1):42.