ACHAIKI IATRIKI | 2025; 44(4):213–220
Case Report
Dimitrios Bousis1, Vasileios Karamouzos1, Virginia Mplani1, Katerina Zafeiri1, Alexandra Georgakopoulou1, Sotiria Kefala1, Aggeliki Bellou1, Eirini Zarkadi1, Markos Marangos2, Fotini Fligou1
1Intensive Care Unit, University Hospital of Patras, Rion, Patras
2Clinic of Internal Medicine, University Hospital of Patras, Rion, Patras
Received: 30 Jun 2025; Accepted: 06 Oct 2025
Corresponding author: Dimitrios Bousis, Intensive Care Unit, University Hospital of Patras, Rion, Patras, Tel.: +30 6972056353, e-mail: dimitrisbousis@gmail.com
Keywords: Meningococcal sepsis, meningococcal meningitis, Neisseria meningitidis serogroup B, purpura fulminans, septic cardiomyopathy
Abbreviations: IV, Intravenous; ER, Emergency Room; CT, Computer Tomography; CSF, Cerebrospinal fluid; gr , Grams; mg, milligrams; pg, Picograms; μg, Micrograms; ICU, Intensive Care Unit; LV, Left ventricle; EF, ejection fraction; Kg, Kilogram; μl, Microlitre; ARDS, Acute Respiratory Distress Syndrome; IMD, Invasive meningococcal disease ; ECMO, Extracorporeal membrane oxygenation; RRT, Renal replacement therapy; SCM, Septic cardiomyopathy; CI, Cardiac index; BNP, B-type natriuretic peptide
Abstract
Acute meningitis and septicemia caused by Neisseria meningitidis is a severe bacterial disease with worldwide distribution. In particular, N. meningitidis serogroup B predominantly causes meningitis and less frequently is associated with the more severe form of the disease, which can on some occasions become complicated with rare but critical clinical manifestations such as limb ischemia and septic cardiomyopathy. Herein, we present the case of a 20-year-old patient with serogroup B meningococcal septicemia presenting with extensive purpura fulminans, septic shock and septic cardiomyopathy. The patient was treated with antibiotics, high-dose vasopressors, fluids and finally levosimendan due to cardiac dysfunction and hypoperfusion. The patient’s condition gradually improved with shock resolution and discontinuation of vasopressor support. Unfortunately, the extensive ischemic lesions on lower extremities led to bilateral leg amputation. The aim of this case-report and literature review is to discuss the complications of invasive meningococcal disease, their management and their impact on the overall prognosis of individuals with meningococcal sepsis.
Introduction
Neisseria meningitidis, commonly referred to as the meningococcus, is a Gram-negative bacterium that appears microscopically as diplococcus, due to its tendency to form pairs [1–3]. It is encapsulated by a polysaccharide layer, which serves as the basis for defining the major serological groups of the bacterium. Among these, types A, B, C, W, X, and Y are those that are more frequently associated with invasive meningococcal disease (IMD) [4–6]. Despite significant progress in rapid diagnostic methods, widespread vaccination campaigns, and the availability of effective antibiotics, IMD continues to pose a serious public health threat. In Europe alone, there were 1,149 confirmed cases and 110 deaths reported in 2022 [7]. The clinical presentation of the disease is typically divided into two main forms: the hemodynamic (sepsis) and the neurological (meningitis) [8]. Due to the severity of these conditions, patients often require admission to the intensive care unit (ICU) for close monitoring and supportive management.
In this narrative review, we discuss a series of rare complications that influenced the clinical course and ultimately defined the outcome of a young patient admitted to our ICU, with meningococcal purpura fulminans.
Case Presentation
A 21-year-old female patient, with no known prior medical history and previously unvaccinated against Neisseria meningitidis serotype B, was admitted to the emergency room (ER) of a University Hospital in Greece, after being found unconscious and febrile (up to 40o C). In the ER, she was tachycardic and hemodynamically unstable, necessitating the administration of IV fluids and vasopressors (noradrenalin 0.17 mcg/kg/min and vasopressin 0.07 units/min) to maintain a mean arterial pressure of 65 mmHg. No signs of meningism were evident, likely due to her reduced level of consciousness (GCS 8/15). Her skin exhibited a diffuse maculopapular hemorrhagic rash that did not blanch upon the application of local pressure (Figure 1). The upper and lower extremities were cyanotic and cold. Laboratory investigations indicated multiple-organ damage: elevated liver enzymes, troponin (1478 pg/ml), serum urea, creatinine, prolonged prothrombin, thromboplastin time (PT/ PTT respectively) and severe thrombocytopenia. The patient was immediately intubated and administered an initial dose of 2 gr ceftriaxone IV. No abnormalities were detected in the brain computer tomography (CT) scan. Subsequently, a lumbar puncture was performed. The overall examination of the cerebrospinal fluid (CSF) was indicative of acute bacterial meningitis (decreased CSF glucose and elevated protein levels), although the CSF culture returned negative. However, blood cultures obtained prior to ceftriaxone administration were positive with N. meningitidis serogroup B. Consequently, individuals who had close contact with the patient within the last 24 hours preceding her admission, and/or worked at the academic institution she attended received chemoprophylaxis with 400 milligrams (mg) ciprofloxacin orally, in accordance with current meningococcal prevention guidelines [9,10].
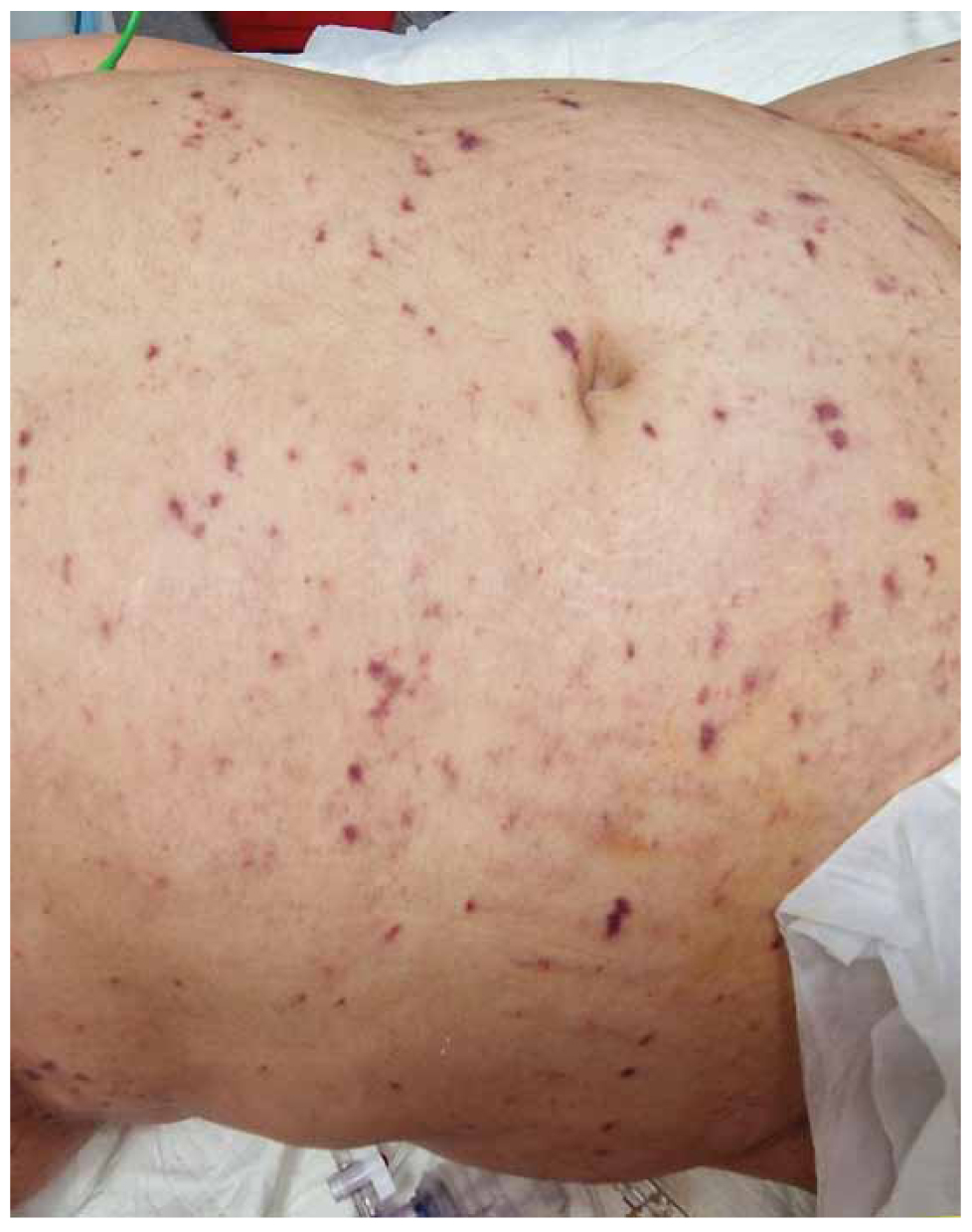
Figure 1. Abdominal purpuric lesions at the time of ER admission.
Within hours of her ER admission, the patient was transferred to the ICU. While being persistently febrile under IV antibiotic treatment with meropenem [2 grams (gr) every eight hours] and vancomycin (1gr twice daily), vasopressor support escalated, and IV hydrocortisone (50mg every six hours) was added to the norepinephrine/vasopressin regimen. The patients’ laboratory troponin values increased dramatically, from 1478 picograms (pg)/ml at the time of admission to over 300,000 pg/ml five hours later. The electrocardiogram (ECG) revealed ST elevation in multiple leads (V2-V6, II, III, a VF). Transthoracic ultrasound indicated an ejection fraction (EF) of 15-20%, global hypokinesia and impaired contractility of the left ventricle (LV), with no evidence of pericardial fluid accumulation. Consequently, IV levosimendan was initiated at a dose of 0.08 μg/min/ kilogram (kg) of bodyweight and the patient underwent coronary angiography with IV contrast medium, which revealed no obstruction or atheromatosis of the coronary arteries. Three hours after the initiation of IV levosimendan, the EF was measured at 35-40% on a subsequent transthoracic cardiac ultrasound. Overall, hemodynamic stability was restored after 24 hours of IV levosimendan and troponin levels gradually decreased over the following days, while the ECG abnormalities also gradually resolved. Intravenous vasopressors were discontinued. On the third day of hospitalization, the EF normalized to values over 50%; however, pericardiac fluid up to 0.8 cm in diameter was detected on multiple cardiac ultrasound examinations. Consequently, hydrocortisone was discontinued and colchicine (0.5 mg twice daily) along with ibuprofen (600 mg every 8 hours) was initiated.
The laboratory abnormalities associated with multiple-organ failure gradually returned to normal. Additionally, no evidence of acquired immune deficiency was detected: the HIV test was negative and complement component levels (ch50 test was performed) were within normal the range.
The appearance of the lower extremities did not improve over time. Despite the gradual improvement of the purpuric skin lesions and the slow normalization of coagulation parameters, the hands and feet remained cyanotic and cold, with a difficult-to-detect pulse on the peripheral arteries. Over time, hands returned to normal, but both feet did not show clinical improvement (Figure 2). In accordance with the guidelines on the management of meningococcal purpura fulminans, despite the thrombocytopenia observed the first four days after admission (approximately 50,000/microlitre (μl) platelets), prophylactic regimen with low molecular weight heparin was administered in an attempt to prevent irreversible occlusion of peripheral arteries [11–13]. Multiple doppler ultrasound examinations of peripheral arterial circulation detected active blood circulation bilaterally in the popliteal, radial, ulnar and dorsalis pedis arteries. Further investigation with CT angiography of the abdominal aorta on the fifth day of hospitalization revealed no obstruction in blood supply below the level of abdominal aorta.
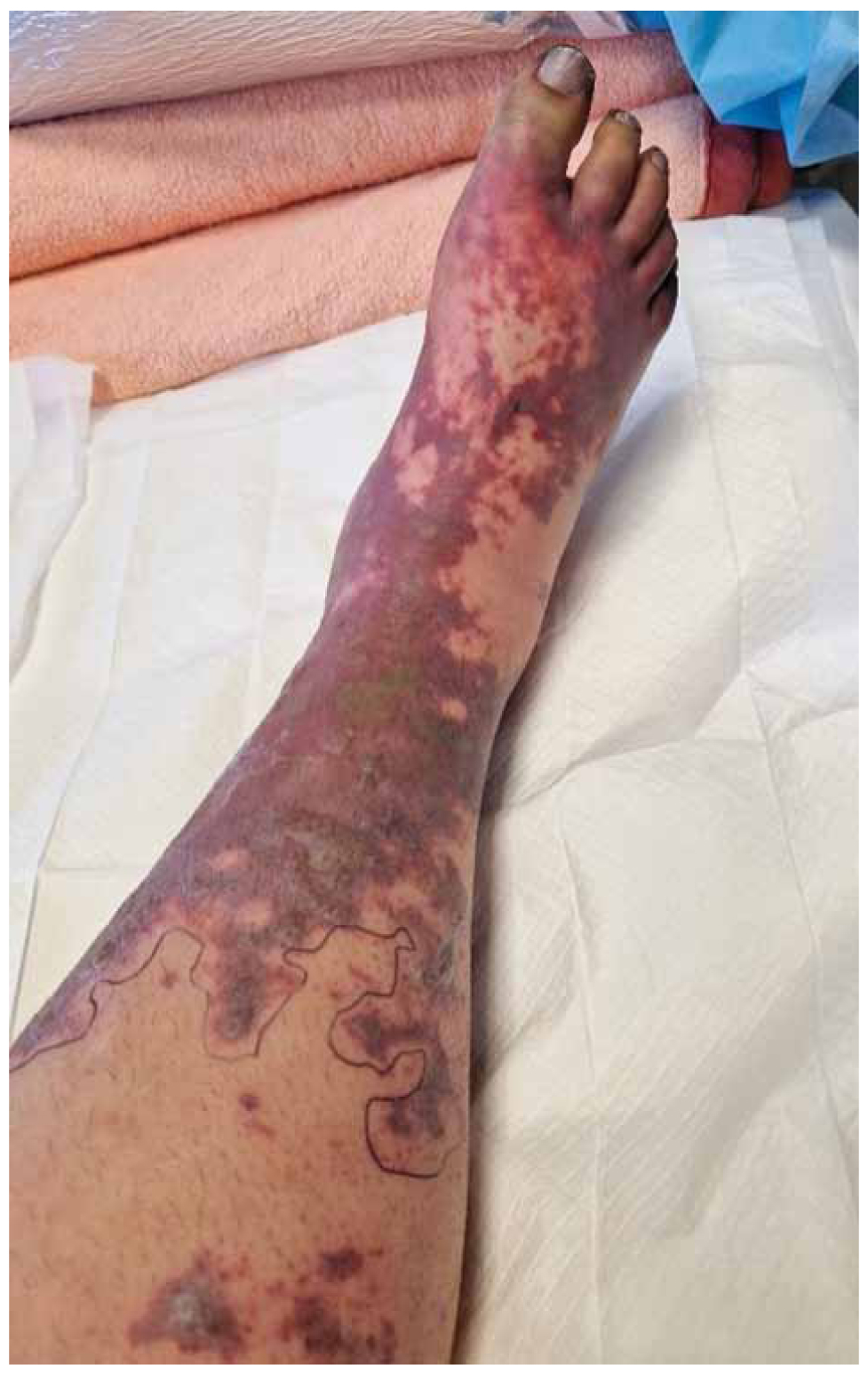
Figure 2. Purpuric and ischemic lesions on the right lower limb.
Following hemodynamic stabilization, the patient developed acute respiratory distress syndrome (ARDS) on the fifth day of hospitalization, necessitating prone position for 12 hours and a modification of the antibiotic regimen to ceftazidime/avibactam IV and colistimethate (both IV and inhaled). Despite these challenges, the patient was successfully extubated on the ninth day and was transferred to the Department of Internal Medicine of our Hospital. Upon the successful completion of the full antibiotic scheme, she was relocated to a specialized center for the treatment of lower limb lesions. However, the clinical condition of her legs did not improve, ultimately resulting in bilateral amputation below the knees.
Meningococcal disease
Neisseria meningitidis is an obligate human pathogen [5], with the human nasopharyngeal mucosa serving as the sole known ecological niche of N. Meningitidis [14, 15]. Since humans are the only natural host for N. meningitidis, no ideal experimental animal models exist regarding the development of IMD [5].Colonization of the nasopharynx by meningococci is a common occurrence in all age groups, with a peak in incidence observed in adolescents and young adults up to 23 years of age. The overall colonization rate is estimated at approximately 8 –10% of the overall population [1,15–17], with a peak prevalence of 23.7% at the age of 19-years [17].
However, on rare occasions, N. meningitidis can evade innate mucosal immunity and progress to a rapidly deteriorating clinical syndrome. This syndrome is characterized by the swift dissemination and proliferation of the bacterium within the bloodstream, leading to colonization of peripheral blood vessels. Subsequently, the bacterium may migrate to the central nervous system by crossing the blood-brain barrier [2,14,18]. The precise etiology underlying this severe clinical syndrome in certain individuals remains unclear. The literature suggests that susceptible human carriers may harbour distinct phylogenetic meningococcal groups with increased virulence compared to asymptomatic carriers. Additionally, genetic polymorphisms in the genomes of patients who develop invasive meningococcal disease have been implicated [14].
Overall, the structural characteristics of N. meningitidis endow it with numerous mechanisms to evade innate immunity and facilitate meningococcal migration and survival within the bloodstream. Notably, bacterial type IV pili, which adhere to human CD46 [4,5,19] and CD147 [14,18] promote adhesion to the nasopharyngeal mucosa and endothelial cells in peripheral vessels. Furthermore, the bacterial factor H binding protein recruits factor H, a component of the complement activation cascade and along with the bacterial NaIP (a serine protease), inhibits the host’s complement activation and deposition of C3b on the meningococcal surface, thereby enhancing survival within human blood vessels [5]. Additionally, N. meningitidis is known to increase iron intake from its human host [15], thereby evading intracellular oxidation after macrophage phagocytosis by metabolising L-glutamate to glutathione [14]. Moreover, by binding to endothelial β2-adrenergic receptors, N. meningitidis induces structural alterations in the endothelial cytoskeleton on the apical membrane of the human endothelial cells and surrounding trans endothelial junctions. This process facilitates the formation of shear-stress- resistant growing bacterial aggregates, such as biofilm, on the apical surface of the endothelium and the gradual development of progressive endothelial leakage into surrounding tissues. This phenomenon primarily affects small peripheral blood vessels and is considered a critical initial step in the progression to invasive meningococcal septicemia [14, 18, 20]. This mechanism enables the bacterium to reach and traverse the blood-brain barrier. Experimental models involving immune-suppressed mice transplanted with human skin grafts, infected subsequently with N. meningitidis, have demonstrated that colonization of human endothelial cells is a necessary precursor to the spread of meningococcal infection to the animal host [20]. Finally, bacterial lipopolysaccharide induces massive activation of the host’s immune system, leading to septic shock and multiple-organ failure [14].
Interestingly, although the phase of progressive bacteremia can be completely clinically asymptomatic [14,18], some patients might develop a diffuse purpuric rash due to extensive endothelial damage, which is subsequently complicated with pathologic activation of the coagulation cascade, ultimately resulting in thrombosis, most evident in peripheral blood vessels and capillaries. This severe form of disseminated intravascular coagulation (DIC) is known as purpura fulminans and is frequently associated with immune deficiencies and/or genetic protein C and S deficiency, which can also arise as an acquired consequence of the meningococcal sepsis itself [21–24]. Purpura fulminans is associated with a poor prognosis [21,23].
Discussion
Neisseria meningitidis rarely progresses from mere saprophytic mucosal colonization of the human nasopharynx [14,18] to IMD, which includes meningitis and septicemia complicated with multiple-organ failure [4,25], with a case-fatality rate ranging from 10% to 40% [26]. IMD can be classified as a “rare disease” according to the actual global definition of this term, which categorizes conditions affecting fewer or equal to one person out of 2000 [27,28].
It is known that the capsule of N. meningitidis serogroup B is significantly less immunogenic compared to the other predominant serogroups, because its polysaccharide layer mimics the molecular structure of human sialic acid and neural cell adhesion molecules [5]. Interestingly, it accounts for the majority of IMD cases worldwide [4,16,26,29–31], in all age-groups [7,32], with reports dating back from the 1960’s [30]. In alignment with global trends, in Greece the majority of meningococcal isolates [33–35] and meningococcal disease cases from 2004 to 2024, about 77.6%, were attributed to N. meningitidis serogroup B [36], while further information regarding the incidence of IMD is limited.
In a recent retrospective study by Contou et al., conducted between 2016 and 2024 in 102 French ICUs, 654 patients were admitted with confirmed IMD. Among these, 62% had meningitis and 38% sepsis. In patients with neurological presentation, serogroup B was predominant, whereas serogroup W135 was common in those with hemodynamic presentation. Patients with sepsis compared to those with meningitis had a lower EF on admission and required more organ support (mechanical ventilation, vasopressors, extracorporeal membrane oxygenation (ECMO), renal replacement therapy (RRT). In-hospital mortality was 4.7% among patients with meningitis and 26.3% among those with sepsis. Among sepsis patients, 20.6% received dobutamine, and limp amputation occurred in 14.8% of hospital survivors [8].
In an older review published by Dastouri F. et al. in 2015, the incidence of limb amputations in IMD survivors was estimated approximately at 2.3% [37]. However, in the subgroup of patients presenting with meningococcal purpura the chances of amputation are higher. Purpura fulminans is characterized by diffuse hemorrhagic skin lesions and focal areas of cutaneous necrosis [21] and is associated with severe dysfunction of the innate coagulation mechanism [11]. N. meningitidis and Streptococcus pneumoniae are the most frequent bacterial triggers [21,38]. Although it is considered a direct complication of meningococcal septicemia, it may also represent a separate clinical entity, still closely associated with IMD, based on pathologic findings featuring excessive white blood cell infiltration of peripheral blood vessels and capillaries, which does not occur in purpura fulminans under other clinical circumstances [11,13]. Overall, in patients presenting directly with purpura fulminans, the risk of limb amputation has been reported to be as high as 28.3%, with one-third of these patients being at risk of losing three-quarters of their hand and feet [22–24,39, 40]. Interestingly, the level of skin demarcation and the overall superficial spread of skin necrosis do not necessarily correlate with or indicate the level of amputation that a patient with purpura fulminans might require [41]. Managing purpura fulminans presents a complex challenge, requiring a delicate balance between treating thrombosis and controlling bleeding risks. Patients at low risk of bleeding require heparin and support with blood products (fresh frozen plasma, red blood cells, cryoprecipitate and platelets) [42].The most recent guidelines from the Japanese Society on Thrombosis and Hemostasis recommend the administration of antithrombin and recombinant thrombomodulin [43].
On the other hand, septic cardiomyopathy (SCM), frequently followed by pericarditis, has been the subject of extensive research and usually is underrecognized. It occurs in approximately 28.2% of all cases of septicemia [44], and is thought to result from the toxic effects of various inflammatory mediators and chemokines, combined with pathological β1-adrenergic signaling. This leads to contractile dysfunction and often increased myocardial cell apoptosis [44–46]. To this date, no clear definition or guidelines for the treatment of SCM exist. The suggested approach is to treat sepsis, restore organ perfusion and this will lead to myocardial function improvement. In those patients in septic shock, cardiac dysfunction and hypoperfusion, the “surviving sepsis campaign” guidelines recommend using epinephrine or adding dobutamine to norepinephrine [47]. The combination of septic and cardiogenic shock leads to five distinct patterns, septic shock, septic shock with sepsis induced cardiogenic shock, septic shock on underlying myocardial dysfunction, cardiogenic shock with superimposed septic shock and pure cardiogenic shock. Furthermore, sepsis induced cardiogenic shock can affect the left, the right or both ventricles changing the therapeutic approach [48]. The literature suggests including transthoracic cardiac ultrasound as a standard diagnostic procedure in patients admitted with purpura fulminans, even in the absence of prior cardiologic examination [11]. However, the effects of meningococcal septicemia on the myocardium have been discussed in only a limited number of case-reports presented on Table 1. Levosimendan, a novel inotropic agent that enhances the sensitivity of intracellular tropomyosin C to calcium, thereby improving myocardial contractility, increasing EF, reducing the risk of arrhythmia and promoting local vasodilation within the myocardium, has been considered a potential new therapeutic approach in septic cardiomyopathy. It has been shown to increase blood supply to internal organs, decrease serum troponin and lactic acid levels and facilitate successful weaning in intubated patients [49]. A recent randomized control trial comparing levosimendan to dobutamine in patients with SCM concluded that patients in the levosimendan arm after 72 hours had significantly higher cardiac index (CI), EF and lower levels of B-type natriuretic peptide (BNP) and troponin [50]. However, the limited size of the existing trials does not provide sufficiently strong evidence to establish the beneficial role of levosimendan in septic cardiomyopathy [50,51].
Currently, two vaccines have been developed, targeting primarily the bacterial factor H binding protein, each for different age groups and have been proven effective against serogroup B N. meningitidis [29,33,34,52,53]. Their use has been endorsed globally for IMD prevention. As of the time of this writing though, vaccination rates have been declining since the onset of the COVID-19 pandemic, resulting in a concurrent increase in new IMD cases worldwide despite an initial significant decline at the beginning of the quarantine period [54]. The patient in this case was unvaccinated against N. meningitidis serogroup B; however, until 2025 vaccination was recommended only for other predominant meningococcal serogroups in the Greek immunization schedule.
In conclusion, IMD caused by meningococcus serogroup B, although a rare disease, poses a serious health problem worldwide, often resulting in severe disability and death. Myocardial dysfunction and limb ischemia requiring amputation are rare complications with significant impact on patient outcome and quality of life. Therefore, even if infrequent, they should always be considered by physicians in order to achieve optimal clinical outcomes.
Conflict of Interest Disclosure
None to declare.
Declaration of Funding Sources
None to declare.
Author Contributions
DB, VK conceived the idea and topic of this article; DB developed the structure and wrote the main body of the article; VK, VB, KZ and AG conducted the search of literature and verified the references mentioned in this article. All authors discussed the content of the article and contributed to its final form.
References
- Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20.
- Linder KA, Malani PN. Meningococcal Meningitis. JAMA. 2019;321(10):1014.
- Meningococcal Diseases – Infectious Diseases – MSD Manual Professional Edition n.d. Available from: https://www.msdmanuals.com/professional/infectious-diseases/gram-negative-cocci-and-coccobacilli/meningococcal-diseases. Accessed 2025 Oct 1.
- Strelow VL, Vidal JE. Invasive meningococcal disease. Arq Neuropsiquiatr. 2013;71(9B):653–8.
- Pizza M, Rappuoli R. Neisseria meningitidis: Pathogenesis and immunity. Curr Opin Microbiol. 2015;23:68–72.
- Chhabria D, Anjankar A. An Overview of Meningococcal Disease’s Recent Diagnostic and Treatment Model. Cureus. 2023;15(11):e48509.
- Invasive meningococcal disease – ECDC Annual Epidemiological Report for 2022 2023. Available from: https://www.ecdc.europa.eu/en/publications-data/invasive-meningococcal-disease-annual-epidemiological-report-2022. Accessed 2025 Oct 1.
- Contou D, Painvin B, Daubin D, Orieux A, Pirollet H, Cour M, et al. Hemodynamic and neurological presentations of invasive meningococcal disease in adults: a nationwide study across 100+ French ICUs: The RETRO-MENINGO study. Intensive Care Med. 2025;51(9):1587–602.
- Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database of Systematic Reviews. 2006;(4):CD004785.
- Clinical Guidance for Meningococcal Disease | Meningococcal | CDC n.d. Available from: https://www.cdc.gov/meningococcal/hcp/clinical-guidance/index.html. Accessed 2025 Apr 23.
- Contou D, Urbina T, de Prost N. Understanding purpura fulminans in adult patients. Intensive Care Med. 2022;48(1):106–10.
- Asif M, Quiroga L, Lagziel T, Ladd SB, Caffrey J, Asif M, et al. A Multidisciplinary Approach to the Management of Severe Purpura Fulminans in a Burn Center: A Case Series. Cureus. 2019;11(8):e5478.
- Contou D, Sonneville R, Canoui-Poitrine F, Colin G, Coudroy R, Pène F, et al. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44(9):1502–11.
- Coureuil M, Join-Lambert O, Lécuyer H, Bourdoulous S, Marullo S, Nassif X. Pathogenesis of meningococcemia. Cold Spring Harb Perspect Med. 2013;3(6): a012393.
- Read RC. Neisseria meningitidis; clones, carriage, and disease. Clinical Microbiology and Infection. 2014;20(5):391–5.
- Guedes S, Bricout H, Langevin E, Tong S, Bertrand-Gerentes I. Epidemiology of invasive meningococcal disease and sequelae in the United Kingdom during the period 2008 to 2017 – a secondary database analysis. BMC Public Health. 2022;22(1):521.
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: A systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–61.
- Coureuil M, Bourdoulous S, Marullo S, Nassif X. Invasive meningococcal disease: A disease of the endothelial cells. Trends Mol Med. 2014;20(10):571–8.
- Melican K, Dumenil G. Vascular colonization by Neisseria meningitidis. Curr Opin Microbiol. 2012;15(1):50–6.
- Capel E, Barnier JP, Zomer AL, Bole-Feysot C, Nussbaumer T, Jamet A, et al. Peripheral blood vessels are a niche for blood-borne meningococci. Virulence. 2017;8(8):1808.
- Perera TB, Murphy-Lavoie HM. Purpura Fulminans. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532865/
- Contou D, Sonneville R, Canoui-Poitrine F, Colin G, Coudroy R, Pène F, et al. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44(9):1502–11.
- Contou D, Urbina T, de Prost N. Understanding purpura fulminans in adult patients. Intensive Care Med. 2022;48(1):106–10.
- Davies H, Pannu K, Edwards J, Pittman M, Mukherjee D. Fulminant Neisseria meningitidis septicaemia with purpura fulminans requiring limb amputation. IDCases. 2020;19:e00742.
- Cabellos C, Pelegrín I, Benavent E, Gudiol F, Tubau F, Garcia-Somoza D, et al. Invasive Meningococcal Disease: What We Should Know, Before It Comes Back. Open Forum Infect Dis. 2019;6(3):ofz059.
- Evidence review for long-term complications and follow-up for meningococcal disease. Evidence Review for Long-Term Complications and Follow-up for Meningococcal Disease: Meningitis (Bacterial) and Meningococcal Disease: Recognition, Diagnosis and Management: Evidence Review I2. 2024.
- Operational Description of Rare Diseases – Rare Diseases International n.d. Available from: https://www.rarediseasesinternational.org/description-for-rd/. Accessed 2025 Apr 23.
- Definition of rare disease – NCI Dictionary of Cancer Terms – NCI n.d. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/rare-disease. Accessed 2025 Apr 23.
- Graña MG, Cavada G, Vasquez M, Shen J, Maervoet J, Klint J, et al. Modeling the public health impact of different meningococcal vaccination strategies with 4CMenB and MenACWY versus the current toddler MenACWY National Immunization Program in Chile. Hum Vaccin Immunother. 2021;17(12):5603–13.
- Nuttens C, Findlow J, Balmer P, Swerdlow DL, Htar MTT. Evolution of invasive meningococcal disease epidemiology in Europe, 2008 to 2017. Euro Surveill. 2022;27(3):2002075.
- Pardo De Santayana C, Tin TinHtar M, Findlow J, Balmer P. Epidemiology of invasive meningococcal disease worldwide from 2010-2019: a literature review. Epidemiol Infect. 2023;151:e57.
- Invasive meningococcal disease – ECDC Annual Epidemiological Report for 2021 2022. Available from: https://www.ecdc.europa.eu/en/publications-data/invasive-meningococcal-disease-annual-epidemiological-report-2021. Accessed 2025 Oct 1.
- Tzankaki G, Markou F, Kesanopoulos K, Levidiotou S, Pangalis A, Tsolia M, et al. Phenotypic assessment of Neisseria meningitidis isolates obtained from patients with invasive meningococcal disease in Greece, 1993-2003: Implications for serogroup B vaccines based on PorA serosubtype antigens. Vaccine. 2006;24(6):819–25.
- Tzanakaki G, Hong E, Kesanopoulos K, Xirogianni A, Bambini S, Orlandi L, et al. Diversity of greek meningococcal serogroup B isolates and estimated coverage of the 4CMenB meningococcal vaccine. BMC Microbiol. 2014;14(1):1–7.
- Tzanakaki G, Georgakopoulou T, Xirogianni A, Papandreou A, Deghmane AE, Magaziotou I, et al. First report of meningococcal ciprofloxacin resistance in Greece due to invasive isolates of the sequence type ST-3129. Eur J Clin Microbiol Infect Dis. 2020;39(12):2467–70.
- National Public Health Organization of Greece (EODY). Epidemiological data regarding meningococcal disease in Greece from 2004 to 2024. Ministry of Health, Greece, 2024 n.d. Available from: https://eody.gov.gr/wp-content/uploads/2025/06/miniggitidokokkiki-nosos-2004-2024-gr.pdf. Accessed 2025 Oct 1.
- Dastouri F, Hosseini A, Haworth E, Khandaker G, Rashid H, Booy R. Complications of serogroup B meningococcal disease in survivors: a review. Infect Disord Drug Targets. 2014;14(3):205–12.
- Contou D, de Prost N, Argaud L, Barbier F, Bazire A, Béduneau G, et al. Clinical phenotype and outcomes of pneumococcal versus meningococcal purpura fulminans: a multicenter retrospective cohort study. Crit Care. 2021;25(1):386.
- Asif M, Quiroga L, Lagziel T, Ladd SB, Caffrey J. A Multidisciplinary Approach to the Management of Severe Purpura Fulminans in a Burn Center: A Case Series. Cureus. 2019;11(8):e5478.
- Ennis J, Ahmed O, Khalid M, Boland PA, Allen M. Meningococcal Sepsis Complicated by Symmetrical Peripheral Gangrene: A Case Report. Cureus. 2020;12(7):e9470..
- Singh D, Swann A. Skin Demarcation and Amputation Level for Foot Gangrene Following Meningococcal Septicemia. Foot Ankle Spec. 2013;6(5):384–8.
- Bendapudi PK, Losman JA. How I diagnose and treat acute infection–associated purpura fulminans. Blood. 2025;145(13):1358–68.
- Yamakawa K, Okamoto K, Seki Y, Ikezoe T, Ito T, Iba T, et al. Clinical practice guidelines for management of disseminated intravascular coagulation in Japan 2024. Part 1: sepsis. Int J Hematol. 2025;121(5):592–604.
- Liang YW, Zhu YF, Zhang R, Ye XL, Zhang M, Wei JR. Incidence, prognosis, and risk factors of sepsis-induced cardiomyopathy. World J Clin Cases. 2021;9(31):9452.
- Drosatos K, Lymperopoulos A, Kennel PJ, Pollak N, Schulze PC, Goldberg IJ. Pathophysiology of Sepsis-Related Cardiac Dysfunction: Driven by Inflammation, Energy Mismanagement, or Both? Curr Heart Fail Rep. 2015;12(2):130.
- Poveda-Jaramillo R. Heart Dysfunction in Sepsis. J Cardiothorac Vasc Anesth. 2021;35(1):298–309.
- Rhodes A, Annane D, Opal SM, Sevransky JE, Sprung CL, Douglas IS, et al. Surviving Sepsis Campaign. International Guidelines for Management of Severe Sepsis and Septic Shock. 2013;41(2):580–637.
- Sato R, Hasegawa D, Guo S, Nuqali AE, Moreno JEP. Sepsis-induced cardiogenic shock: controversies and evidence gaps in diagnosis and management. J Intensive Care. 2025;13(1):1–10.
- Tsolaki V, Zakynthinos GE, Papanikolaou J, Vazgiourakis V, Parisi K, Fotakopoulos G, et al. Levosimendan in the Treatment of Patients with Severe Septic Cardiomyopathy. Life (Basel). 2023;13(6):1346.
- Zhao F, Wei H, Lin L, Wang H, Zhang Z, Guo L. Levosimendan versus dobutamine in septic cardiomyopathy: a randomized clinical trial on cardiac function and safety. Front Cardiovasc Med. 2025;12:1641604.
- Radosevich M, Couture EJ, Nabzdyk C. Levosimendan And Septic Cardiomyopathy: A Key That May Have Found Its Lock? J Cardiothorac Vasc Anesth. 2023;37(3):350–2.
- Castilla J, Cenoz MG, Abad R, Sánchez-Cambronero L, Lorusso N, Izquierdo C, et al. Effectiveness of a Meningococcal Group B Vaccine (4CMenB) in Children. New England Journal of Medicine. 2023;388(5):427–38.
- Rivero-Calle I, Raguindin PF, Gómez-Rial J, Rodriguez-Tenreiro C, Martinón-Torres F. Meningococcal Group B Vaccine ForThe Prevention Of Invasive Meningococcal Disease Caused By Neisseria meningitidis Serogroup B. Infect Drug Resist. 2019;12:3169–88.
- Findlow J, Htar MTT, Villena R, Balmer P. Invasive Meningococcal Disease in the Post-COVID World: Patterns of Disease Rebound. Vaccines (Basel). 2025;13(2):165.
- Karamanolis NN, Nikolaidis CG, Gavgiotakis I, Gaki A, Tatsis I, Mika A, et al. Successfully treated myopericarditis and acute heart failure due to severe Neisseria meningitidis infection: a case report. Diagn Microbiol Infect Dis. 2025;112(4):116837.
- Dawson LP, Hare J, Duffy SJ. Myopericarditis with preserved left ventricular function secondary to Neisseria meningitidis. Diagn Microbiol Infect Dis. 2018;92(3):241–4.
- Bouneb R, Mellouli M, Regaieg H, Majdoub S, Chouchène I, Boussarsar M. Meningococcemia complicated by myocarditis in a 16-year-old young man: a case report. Pan Afr Med J. 2018;29:149.
- Steele L, Bechman K, De Barra E, Mackworth-Young C. Meningococcal arthritis and myopericarditis: a case report. BMC Infect Dis. 2017;17(1):751.
- Woudstra OI, Boink GJJ, Winkelman JA, van Stralen R. A Rare Case of Primary Meningococcal Myopericarditis in a 71-Year-Old Male. Case Rep Cardiol. 2016;2016:1297869.
- Taldir G, Parize P, Arvis P, Faisy C. Acute Right-Sided Heart Failure Caused by Neisseria meningitidis. J Clin Microbiol. 2013;51(1):363.
- Nkosi J, Thakrar A, Kumar K, Ahmadie R, Fang T, Lytwyn M, et al. Meningococcal serotype Y myopericarditis. Diagn Microbiol Infect Dis. 2009;63(2):223–7.
- Ejlertsen T, Vesterlund T, Schmidt EB. Myopericarditis with cardiac tamponade caused by Neisseria meningitidis serogroup W135. Eur J Clin Microbiol Infect Dis. 1988;7(3):403–4.