ACHAIKI IATRIKI | 2021; 40(1):7–10
Editorial
Evanthia Tourkochristou1,2, Athanasia Mouzaki1,3
1Division of Hematology, Department of Internal Medicine, Medical School, University of Patras, Patras, Greece
2Division of Gastroenterology, Department of Internal Medicine, Medical School, University of Patras, Patras, Greece
3Laboratory of Molecular Diagnosis of Infectious Agents, Medical School, University of Patras, Patras, Greece
Received: 26 Dec 2020; Accepted: 23 Jan 2021
Corresponding author: Athanasia Mouzaki, Laboratory of Molecular Diagnosis of Infectious Agents, Medical School, University of Patras, Patras, GR-26500, Greece, Tel.: +30 2610 969123, E-mail: mouzaki@upatras.gr, ORCID: 0000-0001-5548-7002
Key words: SARS-CoV-2, BNT162b2, mRNA-1273, mechanism, clinical trials
Preface
The outbreak of coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in Wuhan, China, spread rapidly around the world and was declared a pandemic by the World Health Organization on March 11, 2020. To date, 97.5 million cases and 2,089,460 deaths have been reported worldwide (Johns Hopkins Coronavirus Resource Center. COVID-19 Map. Accessed January 22, 2021. https://coronavirus.jhu.edu/map.html). The high transmissibility of SARS-Cov-2, which causes different clinical symptoms that escalate to severe, fatal infection in certain cases, made the rapid development of vaccines urgent [1]. The development of mRNA vaccines against SARS-Cov-2 was a strategy that gained rapid acceptance because their production is simple, rapid, and inexpensive – features that can save time against the rapidly spreading COVID -19 pandemic [2].
Previous attempts for mRNA vaccine creation and use
mRNA vaccines have already been designed and tested in preclinical studies for a variety of infectious diseases such as Zika, influenza, papilloma, CMV, Ebola, rabies and HIV viruses, with varying results [4]. mRNA vaccines against chikungunya, hMPV/PIV3, H10N8, and H7N9 viruses were tested in phase I clinical trials and were found to be safe and well tolerated, eliciting robust antibody responses and causing mild side effects at the injection site and systemically [3-5]. A rabies virus glycoprotein (RABV-G) mRNA vaccine caused mild to moderate injection site side effects in almost all vaccinated subjects, while systemic side effects such as fever, fatigue, and pain occurred in 78% of subjects. Nevertheless, the vaccine failed to induce a sufficient immune response as antibody titers dropped 1 year after vaccination [6]. Lower antibody titers were also observed in clinical trials than in animal studies when influenza virus-specific mRNA vaccines were used [5]. An mRNA vaccine against HIV was studied in a phase I trial in chronically HIV-infected patients; the vaccine induced moderate HIV-specific T-cell responses and mild side effects [7]. Studies on optimal dosage, delivery systems and routes of administration are still ongoing to improve the immunogenic efficacy of mRNA vaccines and ensure their good safety profile.
Formulation and action of mRNA vaccines
A conventional mRNA-based vaccine is prepared in a simple, rapid, and effective manner in vitro by transcribing linear plasmid DNA using a T7, a T3, or a Sp6 phage RNA polymerase. The transcript, which consists of an open reading frame encoding the target protein, flanking untranslated regions UTRs and a poly(A) tail, is capped at the 5’ end and purified.
Another type of mRNA platform, called self-amplifying mRNA (samRNA), is based on an engineered genome of alphaviruses that contains genes encoding the target antigen and RNA replication machinery [8]. samRNAs are able to direct their intracellular self-replication, resulting in the synthesis of multiple copies of the target protein in a manner that mimics the de novo production of viral antigens [8]. Lipid nanoparticles (LPNs) are effective transporters for engineered mRNA sequences, ensuring their stability and integrity; however, it is still unclear whether their components can be toxic [9]. A proposed mechanism of mRNA vaccines is shown in Figure 1. Once the LPN-encapsulated mRNA enters the cytosol of an antigen-presenting cell (APC) via endocytosis, it uses the host cell’s translational machinery to drive translation of the target protein, which then undergoes post-translational modifications to become a properly folded, functional protein. The translated target antigen is either degraded by the proteasome within the cell, where the antigen peptides are transported into the endoplasmic reticulum and loaded onto MHC molecules presenting them on the cell surface to induce antigen-specific T-cell responses, or secreted by the host cell and re-captured by other APCs (macrophages, dendritic cells, B-cells). The vaccine mRNA is eventually degraded by the natural intracellular processes [10].
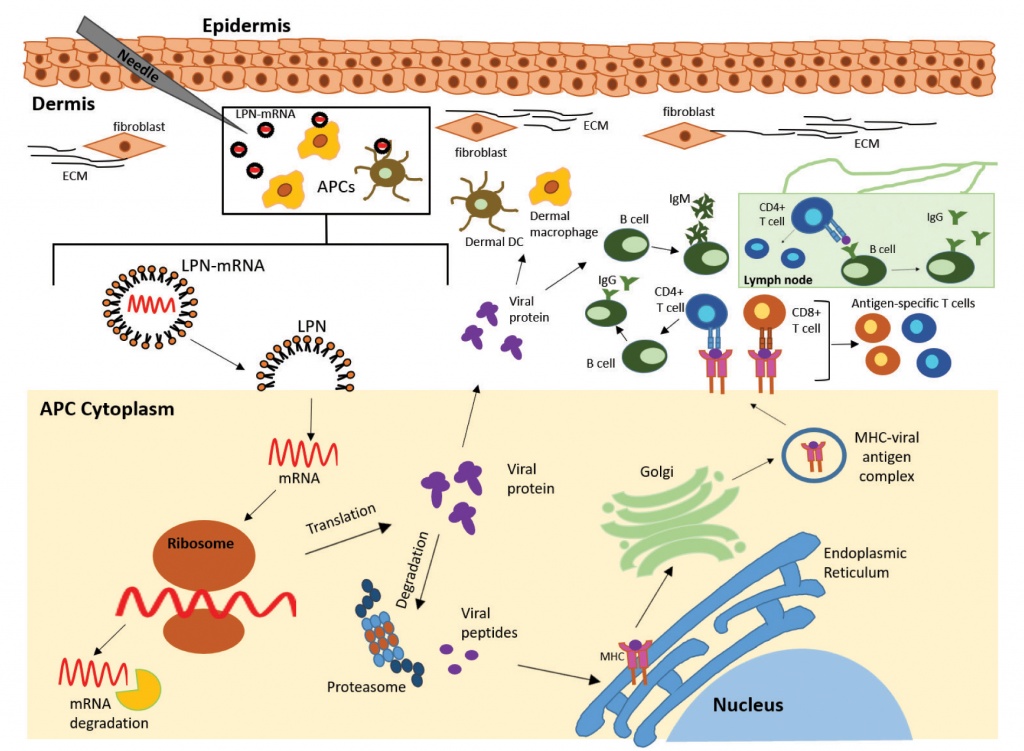
Figure 1. Proposed mechanism of antiviral mRNA vaccine. Intramuscular injection of the vaccine releases mRNA (LPN-mRNA) encapsulated in lipid nanoparticles in the dermis. The LPN-mRNA is taken up by antigen-presenting cells (APCs), including dermal dendritic cells (DCs) and dermal macrophages. Once the LPN-mRNA enters the cytoplasm of the APC by endocytosis, it uses the host cell’s translational machinery to direct the translation of viral proteins (antigens). After synthesis, the viral proteins are properly folded and made functional by post-translational modifications. The translated viral antigens are then degraded by the proteasome within the cell, resulting in antigenic peptides. The peptides are transported into the endoplasmic reticulum and loaded onto MHC (HLA) molecules and presented on the cell surface to naive CD4+ T helper and CD8+ T cytotoxic cells, which become activated antigen-specific T-cells. Antigenic peptides secreted by the host cell are recaptured and re-presented by other APCs. In parallel, they are recognized by naive B-cells in the periphery, which are activated and differentiate into IgM-producing plasmocytes. B-cells that capture antigenic peptides and present them to antigen-specific T helper cells are licensed by these to differentiate and produce isotype-switched antigen-specific antibodies (mainly IgG) in the lymph nodes.
mRNA vaccines against COVID-19
Two samRNA vaccines against SARS-Cov-2, the BNT162b2 (Biotech/Pfizer) and the mRNA-1273 (Moderna), have reached phase III clinical trials. The mRNA-1273 is an LNP-encapsulated mRNA vaccine that encodes the S-2P antigen through which SARS-Cov-2 enters the host cell. This is the first SARS-Cov-2 vaccine to enter phase I clinical trials on March 16, 2020. mRNA-1273, administered in 2 doses, showed mild to moderate adverse effects after both vaccinations, eliciting time- and dose-dependent antibody responses against the S-2P protein, antigen-specific CD4+ T-cell responses, and to a lesser extent CD8+ T-cell responses [11]. Neutralizing antibodies were observed in all participants only after the second vaccination, highlighting the need for administration of two doses. Induction of antibody and Th1 T-cell responses represents a major goal for SARS-CoV-2 vaccines to reduce the risk of vaccine-associated enhanced respiratory disease observed in previous animal studies of SARS-CoV and MERS-CoV infections [12]. A phase III study of 30,420 participants (aged 18 to 65 years) who received the vaccine in two intramuscular doses showed that the efficacy of the vaccine against COVID-19 starting 14 days after the second dose was estimated to be 94.1%, although lower in older participants (>65). Mild local reactions and transient moderate-to-severe systemic events, including fatigue, myalgia, arthralgia, and headache, as well as a low frequency (<2%) of non-fatal serious adverse events and hypersensitivity reactions were observed after the second dose of mRNA-1273 [13].
However, there are still limitations, such as the short follow-up time of the safety and efficacy of the vaccine, as the study is not yet completed and a follow-up of 2 years will be conducted. The FDA granted an Emergency Use Authorization (EUA) for the use of Moderna vaccine mRNA-1273 in adults over 18 years of age in the United States on December 18, 2020, and the European Commission approved the use of Moderna vaccine mRNA-1273 in adults over 18 years of age in Europe on January 6, 2021.
BNT162b2 (BioNTech/Pfizer) is another LPN-formulated mRNA vaccine encoding a prefusion-stabilized membrane-anchored full-length SARS-CoV-2 spike protein. Two phase I/II clinical trials were conducted in the United States and Germany to evaluate the safety and immunogenicity profile of a 2-dose regimen (1, 10, 20, or 30 μg per dose) of BNT162b2 in healthy participants aged 19 to 85 years [14,15]. Two doses of 30μg BNT162b2 achieved a high production of SARS-Cov-2 neutralizing antibody titers (lasting up to 63 days after the boost vaccination) and elicited antigen-specific CD8+ T-cell and Th1-type CD4+ T-cell responses. However, lower antibody responses were observed in older compared to younger participants, a result also observed in other vaccines, and is attributed to immune senescence [16,17]. BNT162b2 entered phase II/III studies (NCT04368728) including 43,448 participants 16 to 55 years or older, assigned 1:1 to receive an intramuscular injection of 30 μg BNT162b2 or placebo in a 2-dose schedule, 21 days apart. A total of 37,706 participants from various ethnic groups, including individuals with obesity and coexisting conditions, and with no evidence of existing or prior SARS-CoV-2 infection were studied for at least 2 months post-vaccination. The efficacy of BNT162b2 was 95% because only 8 cases got COVID-19 among the 36,523 participants after the 2nd dose of vaccination compared to 162 cases that received the placebo [18]. During the clinical evaluation of BNT162b2, a good safety profile was observed, characterized by mild to moderate local reactions in all participants (predominantly injection site pain and injection site redness or swelling, which occurred less frequently) that resolved within 1 to 2 days and did not recur after the second dose. Systemic events (fatigue, headache, chills, fever, muscle/joint pain) after both vaccinations, occurred on day 2 after vaccination and resolved within 5 days. Fever (>38oC), fatigue, and headache were more common in younger participants (16 to 55 years old) than in older ones (>55 years old) and mainly after the second dose of vaccination. A transient slight increase in CRP level and a decrease in blood lymphocytes were also observed. CRP levels and lymphocyte counts are considered pharmacodynamic markers of the mode of action of RNA vaccines, and the transient decrease in lymphocytes is probably due to the observed redistribution of lymphocytes to lymphoid tissues related to the stimulation of the innate immune system, according to previous clinical data on RNA vaccines [19]. BNT162b2-related serious adverse events were reported in a few participants, including postvaccination shoulder injury, right axillary lymphadenopathy, paroxysmal ventricular arrhythmia, and right leg paresthesia; serious systemic events occurred in less than 4% of participants. Although 2 BNT162b2 and 4 placebo deaths occurred, none were related to vaccine or placebo administration. Safety monitoring will continue for 2 years after the second dose. The encouraging results of the Phase II/III trials allowed the FDA to grant BioNTech/Pfizer an emergency approval for BNT162b2 on December 11, 2020 and the European Commission to grant BioNTech/Pfizer a conditional marketing authorization (CMA) on December 21, 2020.
Conclusions
Future considerations include evaluation of adverse events longer than 2 months after boost vaccination, duration of vaccine protection, vaccine protection in younger adolescents, children, pregnant women, immunocompromised individuals, individuals with asymptomatic infection, and individuals with a history of coronavirus. In addition, ongoing studies need to optimize RNA formulation and stability to reduce the need for cold storage (the vaccine is currently stored and shipped at -60 to -80oC) and improve vaccine efficacy, as the nucleotide composition of RNA may affect its immunostimulatory activity and reactogenicity profile [20].
Overall, given the relatively good safety and immunogenicity profiles of mRNA vaccines, there is optimism that global vaccination will help end the pandemic COVID-19. Hopefully, if this type of vaccine proves effective, there will be renewed efforts to use this technology to produce vaccines for the many infectious diseases that remain incurable.
Conflict of interest disclosure: None to declare.
Declaration of funding sources: ET is a recipient of Karatheodoris grant #80672 from the University of Patras.
References
- Sun K, Chen J, Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population-level observational study. Lancet Digit Health. 2020; 2(4):e201-e208.
- Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020; 5:11.
- Moderna Announces Positive Interim Phase 1 Data for First Combination Vaccine Against the Respiratory Viruses hMPV and PIV3. 2019. Available from: https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-positive-interim-phase-1-data-first
- Shaw C, Panther L, August A, Zaks T, Smolenov I, Bart S, et al. Safety and immunogenicity of a mRNA-based chikungunya vaccine in a phase 1 dose-ranging trial. Int J Inf Dis. 2019; 79(S1):17.
- Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ, et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol Ther. 2017; 25(6):1316-1327.
- Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017; 390(10101):1511-1520.
- Leal L, Guardo AC, Morón-López S, Salgado M, Mothe B, Heirman C, et al. Phase I clinical trial of an intranodally administered mRNA-based therapeutic vaccine against HIV-1 infection. AIDS. 2018; 32(17):2533-2545.
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov. 2018; 17(4):261-279.
- Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016; 7(5):319-334.
- Wadhwa A, Aljabbari A, Lokras A, Foged C, Thakur A. Opportunities and Challenges in the Delivery of mRNA-based Vaccines. Pharmaceutics. 2020; 12(2):102.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA Vaccine against SARS-CoV-2 – Preliminary Report. N Engl J Med. 2020; 383(20):1920-1931.
- Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020; 586(7830):567-571.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2020; (in press). doi: 10.1056/NEJMoa2035389
- Walsh EE, Frenck RW, Jr., Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N Engl J Med. 2020; 383(25):2439-2450.
- Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv. 2020. doi: https://doi.org/10.1101/2020.12.09.20245175
- Muñoz N, Manalastas R, Jr., Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: a randomised, double-blind trial. Lancet. 2009; 373(9679):1949-1957.
- Boraschi D, Del Giudice G, Dutel C, Ivanoff B, Rappuoli R, Grubeck-Loebenstein B. Ageing and immunity: addressing immune senescence to ensure healthy ageing. Vaccine. 2010; 28(21):3627-3631.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N Engl J Med. 2020; 383(27):2603-2615.
- Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006; 108(10):3253-3261.
- Kondili M, Roux M, Vabret N, Bailly-Bechet M. Innate immune system activation by viral RNA: How to predict it? Virology. 2016; 488:169-178.